Hemoglobin is a complex iron-containing protein found in erythrocytes (red blood cells) of most vertebrates that plays a vital role in carrying oxygen and carbon dioxide within the blood.
It is also the component that makes our blood red. It is found in animals containing red-colored blood i.e. in almost every vertebrate except fishes of the Channichthyidae family and in a few invertebrates.
Hemoglobin is abbreviated as Hb or Hgb.
- Beside RBC, hemoglobin is also present in alveolar cells, macrophages, some neurons of the midbrain, mesangial cells of kidneys, hepatocytes, vaginal epithelial and cervical cells, etc.
- Hemoglobin is synthesized together with the red blood cells (RBCs) during erythropoiesis in the bone marrow. The iron part (heme part) is synthesized in the cytoplasm and mitochondria of developing RBC and the protein part (globin protein) is synthesized by the ribosome of the growing RBC. The synthesis of globin is controlled by three different genes; the alpha-globin genes HBA1 and HBA2 and the beta-globin gene HBB.
- Hgb is the main component in blood that transports oxygen and carbon dioxide gases. One Hgb molecule can carry up to 4 molecules of oxygen or carbon dioxide gas molecules at a time. These gases are attached to the heme component of the hemoglobin. When oxygen binds with the hemoglobin, it is called the oxyhemoglobin and about 98% of oxygen is carried in our blood is carried by the oxyhemoglobin. Similarly, when carbon dioxide binds with the hemoglobin, it is called carbaminohemoglobin. Blood carries about 25% of the released carbon dioxide in this form.
Interesting Science Videos
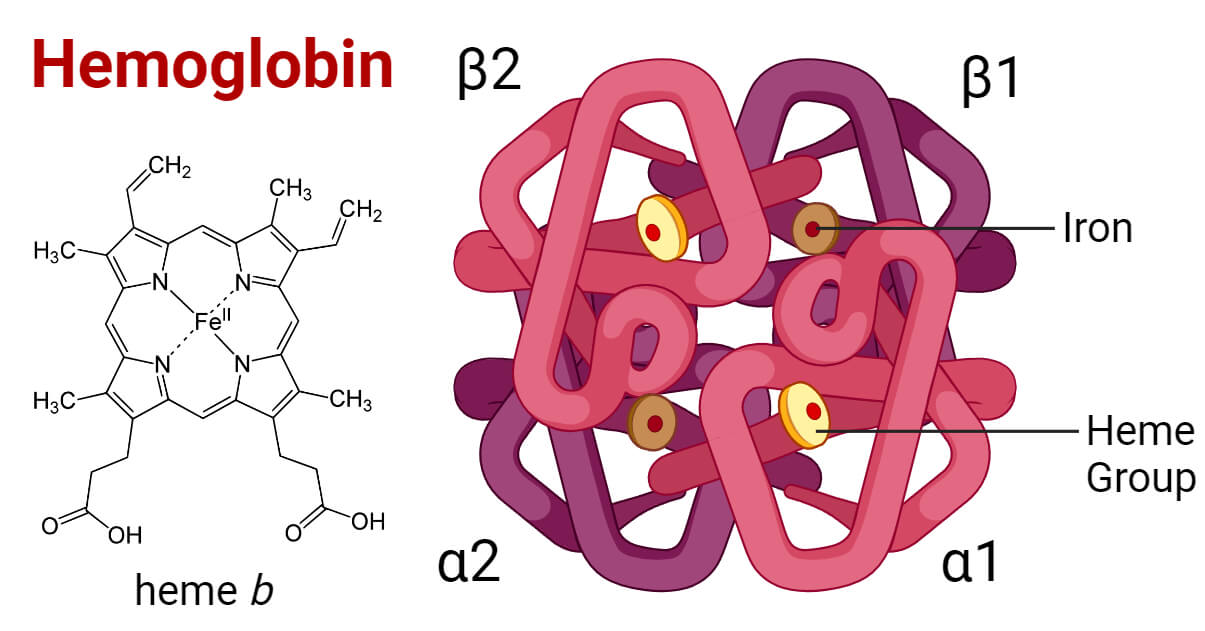
Structure of Hemoglobin
- Hgb is a globular metalloprotein with a quaternary structure. One hemoglobin molecule is composed of four subunits; each containing one polypeptide chain (chain of globin protein) attached with a prosthetic heme group. Each subunit weighs about 16,000 Dalton making the total molecular weight of a Hgb molecule of 64,000 Dalton.
- The polypeptide chains in adults are of two types, the alpha chain and the beta chain containing 141 and 146 amino acids respectively. Adult hemoglobin has two alpha subunits (α1 and α2) and two beta subunits (β1 and β2) combined as two αβ dimers that are arranged around 2-fold axis symmetry. Each subunit has a heme attached to the globin protein. In fetal hemoglobin, the beta subunits are replaced by gamma subunits (γ1 and γ2). In a rare amount of hemoglobin, the beta subunits are replaced by delta subunits (δ1 and δ2).
- The heme group contains an iron (ferrous ion, Fe+2) held in the center of a porphyrin ring binding with the nitrogen atoms of the ring. The Fe+2 ion is bound to the pocket of the globin subunit with a histidine residue. Each Fe+2 ion can bind with one oxygen (O2) molecule or one carbon dioxide (CO2) molecule.
Types of Hemoglobin
Based on the non-alpha subunits, normal hemoglobin is mainly of three types:
- Haemoglobin A
It is the predominant type of hemoglobin accounting for about 95 to 98% of total adult hemoglobin. It contains two alpha subunits and two beta subunits.
- Hemoglobin A2
It accounts for about 2 to 3% of total adult hemoglobin. It contains two alpha subunits and two gamma subunits.
- Hemoglobin F
It is the hemoglobin of fetuses and newborns and it is present in scanty amounts, below 1%, in adults. It contains two alpha subunits and two delta subunits.
Besides these major hemoglobin, there are other mutated forms also like hemoglobin E, hemoglobin S, and hemoglobin C.
Functions of Hemoglobin
The primary function of hemoglobin is to carry oxygen and carbon dioxide gases. It also serves the secondary function of maintaining blood pH and buffering the blood.
- Oxygen Transport
Fe+2 ion of a heme group can bind one O2 molecule; hence, a total of 4 O2 molecules can be carried by one Hgb. When O2 is bound with Hgb, it is called oxyhemoglobin and Hgb without bound O2 is called deoxyhemoglobin. About 98% of oxygen in the blood is carried by oxyhemoglobin.
- Carbon dioxide Transport
Fe+2 ion of a heme group can bind one CO2 molecule; hence, a total of 4 CO2 molecules can be carried by one Hgb. When CO2 is bound with Hgb, it is called carbaminohemoglobin. About 20 to 25% (on average 23%) of CO2 in blood is carried by carbaminohemoglobin.
- Other Gases/Ion Transport
Besides O2 and CO2, Hgb can also bind with other ligands like carbon monoxide (CO), nitric oxide (NO), sulfur monoxide (SO), nitrite ion (NO-2), sulfides (S-2), etc. The affinity of Hgb with CO is more than 200 times the affinity of Hgb with O2. When CO is bound with Hgb, it is called carboxyhemoglobin.
- Regulation of Blood pH and Buffering Function
Hgb molecules can also bind to the hydrogen ions and maintain the pH of the blood.
Normal Hemoglobin Level
The amount of hemoglobin in blood is expressed in grams per deciliter (g/dl). The amount of hemoglobin depends on the age, sex, and health status of an individual. In general, the Hgb level in a human range from 12 to 20+ g/dl.
| Age of Person | Normal Hgb Level (g/dl) |
| Newborn | 14 to 24 |
| 2 weeks | 13 to 20 |
| 3 months | 9.5 to 14.5 |
| 6 months to 6 years | 10.5 to 14.0 |
| 6 years to 12 years | 11 to 16 |
| Adult male | 14 to 18 |
| Adult female | 12 to 16 |
Diseases Related to Hemoglobin
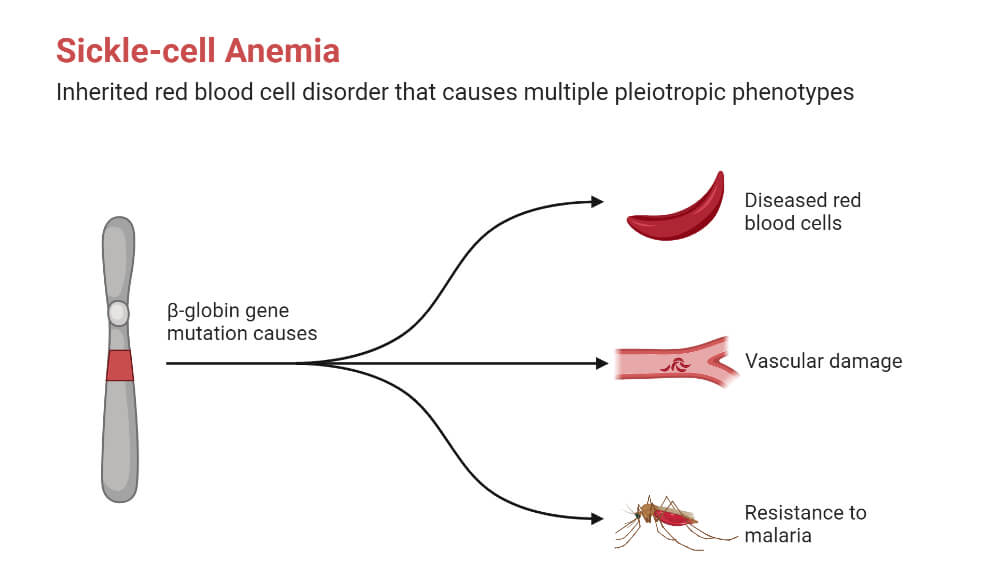
- Sickle Cell Disease
It is the condition when the body produces abnormal hemoglobin, hemoglobin S, due to a mutation in the beta-globin gene HBB.
- Thalassemia
It is an inherited disease characterized by a decrease in hemoglobin production by the body. It is due to the reduction or complete absence of one or more globin subunits.
- Polycythemia
It is characterized by increased hemoglobin levels in the blood.
- Methemoglobinemia
It is the condition characterized by a reduction in the ability of hemoglobin to carry oxygen due to a change of iron from the reduced Fe+2 (ferrous) states to the oxidized Fe+3 (ferric) states.
- Hemoglobinuria
Presence of hemoglobin in urine.
- Hereditary persistence of fetal hemoglobin (HPFH)
It is a benign condition characterized by the presence of fetal hemoglobin, hemoglobin F, in adults in abundant quantity.
References
- Rhodes CE, Denault D, Varacallo M. Physiology, Oxygen Transport. [Updated 2022 Nov 14]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK538336/
- Billett HH. Hemoglobin and Hematocrit. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Boston: Butterworths; 1990. Chapter 151. Available from: https://www.ncbi.nlm.nih.gov/books/NBK259/
- Marengo-Rowe, A. J. (2006). Structure-function relations of human hemoglobins. Proceedings (Baylor University. Medical Center), 19(3), 239-245. https://doi.org/10.1080/08998280.2006.11928171
- Ahmed, M. H., Ghatge, M. S., & Safo, M. K. (2020). Hemoglobin: Structure, Function and Allostery. Sub-cellular biochemistry, 94, 345. https://doi.org/10.1007/978-3-030-41769-7_14
- Wittenberg, B. A., Briehl, R. W., & Wittenberg, J. B. (1965). Haemoglobins of invertebrate tissues. Nerve haemoglobins of Aphrodite, Aplysia and Halosydna. Biochemical Journal, 96(2), 363-371. https://doi.org/10.1042/bj0960363
- Kaufman DP, Khattar J, Lappin SL. Physiology, Fetal Hemoglobin. [Updated 2023 Mar 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK500011/
- Palmeri R, Gupta V. Carboxyhemoglobin Toxicity. [Updated 2023 Apr 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557888/
- Linderkamp, O., Zilow, E. P., & Zilow, G. (1992). Kritische Hämoglobinwerte bei Neugeborenen, Säuglingen und Kindern [The critical hemoglobin value in newborn infants, infants and children]. Beitrage zur Infusionstherapie = Contributions to infusion therapy, 30, 235–264.
- Segel, G. B., Hirsh, M. G., & Feig, S. A. (2002). Managing anemia in pediatric office practice: Part 1. Pediatrics in review, 23(3), 75–84. https://doi.org/10.1542/pir.23-3-75
- Rochette, J., Craig, J. E., & Thein, S. L. (1994). Fetal hemoglobin levels in adults. Blood reviews, 8(4), 213–224. https://doi.org/10.1016/0268-960x(94)90109-0
- Pillai AA, Fazal S, Mukkamalla SKR, et al. Polycythemia. [Updated 2023 May 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526081/
- Ludlow JT, Wilkerson RG, Nappe TM. Methemoglobinemia. [Updated 2022 Aug 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537317/
- Kohne, E. (2011). Hemoglobinopathies: Clinical Manifestations, Diagnosis, and Treatment. Deutsches Ärzteblatt International, 108(31-32), 532-540. https://doi.org/10.3238/arztebl.2011.0532
- Sharma, D. C., Singhal, S., Woike, P., Rai, S., Yadav, M., & Gaur, R. (2020). Hereditary persistence of fetal hemoglobin. Asian Journal of Transfusion Science, 14(2), 185-186. https://doi.org/10.4103/ajts.AJTS_71_16
- Britannica, The Editors of Encyclopaedia. “hemoglobin”. Encyclopedia Britannica, 28 Apr. 2023, https://www.britannica.com/science/hemoglobin. Accessed 9 June 2023.
- https://byjus.com/neet/what-is-haemoglobin/
- https://www.getbodysmart.com/respiratory-gases-and-their-transport/hemoglobin-structure/
- https://www.slideshare.net/NCSLS/hemoglobin-structure-67264726
- https://surendranathcollege.ac.in/new/upload/SUBHADIPA%20MAJUMDER2022-07-22Hemoglobin%20structure.pdf
- https://pdb101.rcsb.org/motm/41
- https://www.ucsfhealth.org/education/hemoglobin-and-functions-of-iron
- https://www.urmc.rochester.edu/encyclopedia/content.aspx?contenttypeid=167&contentid=hemoglobin
- https://www.uptodate.com/contents/image?imageKey=PEDS%2F101544
- https://www.aafp.org/pubs/afp/issues/2016/0215/p270.html
- Hemoglobin. (2023, June 7). In Wikipedia. https://en.wikipedia.org/wiki/Hemoglobin
- Hemoglobin (ucsfbenioffchildrens.org)
- https://www.ucsfhealth.org/medical-tests/hemoglobin-electrophoresis
- https://www.cdc.gov/ncbddd/thalassemia/facts.html
- https://www.nhlbi.nih.gov/health/thalassemia
- https://www.cdc.gov/ncbddd/sicklecell/facts.html
- https://www.nhlbi.nih.gov/health/sickle-cell-disease
- https://www.hopkinsmedicine.org/health/conditions-and-diseases/sickle-cell-disease

Hello there, can you double check regarding the subunit for hba2 (a2d2) and hbf (a2g2)? I think its misplaced.