Neospongodes atlantica, a potential case of an early biological introduction in the Southwestern Atlantic
- Published
- Accepted
- Received
- Academic Editor
- James Reimer
- Subject Areas
- Biodiversity, Ecology, Marine Biology, Taxonomy, Zoology
- Keywords
- Octocorallia, Invasion, Continental shelf, Soft-bottom, Habitat Modelling, Brazil
- Copyright
- © 2022 Cordeiro et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2022. Neospongodes atlantica, a potential case of an early biological introduction in the Southwestern Atlantic. PeerJ 10:e14347 https://doi.org/10.7717/peerj.14347
Abstract
Soft corals (Anthozoa: Octocorallia) are discreet components in the Southwestern Atlantic reef communities. In Brazil, the native octocoral shallow-reef fauna is mostly represented by gorgonians. Consequently, except for the nephtheid Neospongodes atlantica, most of the known soft corals from this region are considered non-indigenous. Hitherto, the monotypic genus Neospongodes, which was proposed in the early 1900s, has been considered to be endemic to the Northeastern Brazilian coast. Herein, based on in situ records, we show that N. atlantica is a substrate generalist that has been probably expanding its distribution by dominating extensive shallow and mesophotic sandy and reef bottoms, generally outcompeting other reef benthic organisms, including Brazilian endemic species. Based on previously unidentified museum specimens, new records, and a broad literature review, we provide the most comprehensive modelling of the potential distribution of this species in the Southwestern Atlantic. Based on molecular inference supported by in-depth morphological analysis, the probable non-indigenous and, therefore, ancient introduction of N. atlantica in Brazilian waters is discussed. Finally, these results support that Neospongodes and the Indo-Pacific Stereonephthya are synonyms, which led us to propose the latter as taxonomically invalid.
Introduction
The Brazilian Exclusive Economic Zone harbors the largest and most diverse coral reef communities in the South Atlantic (Leão, Kikuchi & Testa, 2003; Francini-Filho et al., 2013), including a high percentage of endemic corals (Souza et al., 2017). Brazilian shallow-water reefs have been the focus of most marine assessments to date, including biodiversity surveys and mapping (e.g., Leão et al., 2016). However, in the last decade, ecosystems deeper than 30 m (mesophotic ecosystems) started to be assessed, with most studies concentrated in oceanic islands and the north, northeastern and central Brazilian continental shelfs (Cordeiro et al., 2015; Magalhães et al., 2015; Moura et al., 2016; Pinheiro, Bernardi & Simon, 2017; Francini-Filho et al., 2019; Soares, Tavares & Carneiro, 2018).
Recently, remotely operated vehicle (ROV) surveys have revealed dense monospecific aggregations of the soft coral Neospongodes atlantica Kükenthal, 1903 on soft-bottoms off several localities of the northeastern Brazilian coast, a still undescribed kind of benthic community (Moura et al., 2013). Neospongodes atlantica is the only representative of the genus and, to date, considered endemic to Brazil (Castro, Medeiros & Loiola, 2010). In terms of distributional records, N. atlantica has been reported only to its type locality and the type locality of N. bahiensis (see Kükenthal, 1903 - the later synonymized with the former by Verseveldt (1983)), both representing shallow-reefs from the Bahia State (∼12–16°S). One additional single record was that from Castro, Medeiros & Loiola (2010) at the Rio Grande do Norte State (∼6°S). Overall, octocorals are considered ecosystem engineers that, in some localities, support dense assemblages in shallow and deep-waters, adding tri-dimensionality, modulating water flow and, consequently, increasing the diversity in reef environments (Roberts, Wheeler & Freiwald, 2006; Buhl-Mortensen et al., 2010; Nelson & Bramanti, 2020). Octocorals are reported from all oceans and are ubiquitous in coral communities around the globe, being regarded as one of the main components and great competitors for space in benthic assemblages of the Indian and Central Western Pacific ocean basins (Fleury et al., 2008; Janes & Mary, 2012; Pérez et al., 2016), as well as their rise in the Caribbean during the last decades (Lasker et al., 2020).
In the Southwestern Atlantic, more specifically in the Brazilian coast, octocorals are considered discreet components of the reef communities (see Pérez et al., 2016; Aued et al., 2018), with reduced populations (Cassola et al., 2016) usually concentrated in narrow bathymetric and geographic ranges (e.g., Castro, Medeiros & Loiola, 2010; Francini-Filho et al., 2018). In general, the Caribbean and Brazilian octocoral communities are predominantly composed of gorgonian corals (e.g., families Gorgoniidae and Plexauridae) (Bayer, 1961; Pérez et al., 2016; Sánchez, 2016). On the other hand, the Indo-Pacific octocoral communities are dominated by soft corals, mainly those from the families Alcyoniidae, Xeniidae and Nephtheidae (Fabricius, 1997; Fabricius & Alderslade, 2001). Such biogeographical pattern raises uncertainties on the native condition of nephtheids, such as Neospongodes, in Brazilian waters.
Although other Neospongodes species have been described, it is currently accepted as a monotypic genus (Cordeiro et al., 2021). For example, Neospongodes agassizi (Deichmann, 1936) and Neospongodes caribaea (Deichmann, 1936) were transferred to the genus Chironephthya (Cairns & Bayer, 2009; Imahara et al., 2017), whereas Neospongodes portoricensis (Hargitt & Rogers, 1901) is accepted as Stereonephthya (Verseveldt, 1983). Because of such taxonomical challenges, it is also important to consider the relationship between the Neospongodes and Stereonephthya, the latter common in the Indo-Pacific (Fabricius & Alderslade, 2001; Chanmethakul, Chansang & Watanasit, 2010).
Morphologically, Neospongodes is almost indistinguishable from Stereonephthya in most characters, such as colony and sclerite shape, size, and distribution (Verseveldt, 1983; Ofwegen & Groenenberg, 2007). Also, recent molecular-based phylogenies have added to the previous evidence that there are species complexes that demand more in-depth studies (Ofwegen, 2005; Ofwegen, 2007). Assuming that both Neospongodes and Stereonephthya correspond to a single genus, its occurrence in the Southwestern Atlantic could represent an ancient introduction. Such hypothesis is tested herein in the light of classical morphology and molecular data. In such a context, since the Brazilian unique marine biodiversity is under crescent threats, such as industrial pollution, fisheries, ocean drilling, and bioinvasion (Werner, Pinto & Dutra, 2000; Miranda & Marques, 2016; Creed et al., 2017; Francini-Filho et al., 2018; Capel et al., 2020; Carpinelli et al., 2020; Tanasovici, Kitahara & Dias, 2020), conservation actions are needed to address such challenges (Castro & Pires, 2001; Picciani et al., 2016). Apart from shedding light on N. atlantica taxonomy and systematics, we also modeled its potential habitat (PH) distribution (including the newly found octocoral forest (OF)) through Species Distribution Models (SDMs) (Pearson et al., 2007). Taken together, our results provide the very first tools for decision-makers in terms of the potential invasiveness of this species on the Brazilian coast and discuss on possible ecological consequences of N. atlantica silent spread into Brazilian shallow-reef areas.
Material and Methods
In situ records
Newly discovered and previously reported Brazilian OFs were accessed through non-targeted SCUBA and technical diving, and ROV surveys between 2007 and 2017 in depths of up to 80 m deep at three localities in distinct latitudes. In situ records resulted from occasional findings extracted from not-scaled and not-standardized surveys. For this reason, some information regarding dive/mission are missing, including ROV bottom time. The first survey, at the Equatorial outer continental shelf (3°26′S/38°08′W, off Ceará State), occurred in March 2010 at 61 m deep using a Seabotix ROV, revealing a relatively flat rocky substrate covered by a thin veneer of medium grained sands with about 12% of CaCO3 (Moura et al., 2013; https://figshare.com/articles/media/Supplemental_Video_1_mp4/21534636). Another survey, carried out at the Cabeço Brasil through technical dives on November 2015 (08°13′S/34°36′W, off Pernambuco State), aimed at a mesophotic coral-sponge community between 50 and 75 m at a continental shelf-break paleo-channel. This paleo-channel displayed a central sandstone reef surrounded by soft-bottoms and rhodoliths (https://figshare.com/articles/media/Supplemental_Video_2_mp4/21539517). Finally, the last sets of in situ information gathered using technical and ROV dives were performed at the Abrolhos Bank (17°53′S/18°20′S and 38°40′W/39°10′W, Bahia State) around shallow reef pinnacles (e.g., Timbebas reef) between 18 and 81 m (https://figshare.com/articles/media/Supplemental_Video_3/21539613). Field studies were performed under ICMBio licenses SISBIO 15691-1 and SISBIO-11709-1.
Museum specimens and morphological analysis
Fifty-nine specimens of N. atlantica from the Petrônio Alves Coelho Oceanographic Museum (MOUFPE-CNI) and National Museum (MNRJ) collections (Table S1) were examined to investigate the distribution of OFs in Brazilian waters. Fragments of specimens were dissolved in sodium hypochlorite to allow sclerite examination with a light-microscope and also a scanning electron microscope (Jeol 6460-LV). Identifications followed Bayer (1961) and Bayer, Grasshoff & Verseveldt (1983). A specimen sampled through technical diving off Recife (Pernambuco State, Brazil) was used for DNA extraction.
DNA extraction, amplification and sequencing
Total genomic DNA was extracted using the DNeasy blood and tissue kit (Qiagen, Inc. Valencia, CA, USA) following the manufacturer’s protocol. DNA extraction yield and quality were verified using a spectrophotometer (Nanodrop, Thermo Fisher Scientific, Waltham, MA, USA) and a 1% agarose gel electrophoresis, respectively. Polymerase chain reactions (PCR) cycling of mitochondrial genes followed McFadden et al. (2006) and Cairns & Wirshing (2015), and targeted the genes: mtMutS (msh1); and COI + IGR1 (Table 1). The nuclear long ribosomal gene 28S rDNA was partially amplified according to Halàsz et al. (2015) (Table 1). For each gene, PCR reaction totaled 15 µl containing: 1.5 µl of the 10X PCR buffer; 0.45 µl of MgCl2 (50 mM); 0.3 µl of dNTP (10 mM); 0.3 µl of each primer (10 µM); 11.09 µl of ddH2O; 0.06 µl of Platinum taq DNA Polymerase (0.6 U); and 1.0 µl of the DNA sample. Amplicons were purified according to ExoSAP-IT protocol and sequenced using the AB 3500 Genetic Analyzer in both directions. This study is registered under SISGEN record A7E8638.
Phylogenetic analysis
Sequences were edited and concatenated using Geneious v.2020.2.2 and Fasta Alignment Joiner (Villesen, 2007), and then aligned with nepththeid sequences deposited in GenBank (Table S1) using MUSCLE available at the EMBL-EBI platform (Edgar, 2004; Madeira et al., 2019), and manually refined using Jalview v.2.11.1.0 (Waterhouse et al., 2009). Statistical analyzes were performed using the software MEGA-X (Kumar et al., 2018). Acrophytum claviger (GenBank accession: JX203823.1) was used as an outgroup. The JModelTest2 (Darriba et al., 2012) was used to define the best-fit model of evolution of the resulting nucleotide alignment. Bayesian Inference (BI) of the mtMutS alignment was performed using MrBayes v.3.2.7 (Ronquist & Huelsenbeck, 2003) at the CIPRES platform (Miller, Pfeiffer & Schwartz, 2010) with four parallel runs with 10 million generations each. The first quarter of the sampled topologies were discarded as burnin. For the concatenated alignment, BI was performed in four parallel runs with 4 million generations each and had the same percentage of the sampled trees discarded as burnin. Maximum-likelihood analysis (ML) was performed using the RAxML v.8.2.12 with 1,000 bootstrap replications (Stamatakis, 2014).
| Primers | Sequence 5′–3′ | References | Gene |
|---|---|---|---|
| MSH1-F | AGGAGAATTATTCTAAGTATGG | Herrera, Baco & Sánchez (2010) | mtMuts |
| MSH1-3458R | TSGAGCAAAAGCCACTCC | Sánchez, Lasker & Taylor (2003) | mtMuts |
| CO1-LA-8398F | AATGGCGGGGACAGCTTCGAGTATGTTAATACGG | Brugler & France (2008) | CO1 |
| CO1-OCT-R | ATCATAGCATAGACCATACC | France & Hoover (2002) | CO1 |
| 28S-Far | CACGAGACCGATAGCGAACAAGTA | McFadden & van Ofwegen (2013) | 28S |
| 28S-Rab | TCGCTACGAGTCTCCACCAGTGTTT | McFadden & van Ofwegen (2013) | 28S |
Occurrence, environmental data, and modelling
Besides the examined specimens and video surveys, previously published information on occurrence records (latitude and longitude) (Epifanio et al., 1999; Dutra et al., 2006a; Ferreira et al., 2009; Lima et al., 2013) and also from open data sources (e.g., http://splink.cria.org.br and http://gbif.org) were compiled.
Through simple rarefaction, occurrence data were reduced to one per pixel (∼1 km) of the study area. Pixels that showed environmental data values far from the centroid of the overall values (based on the weighted value of each PC result) were excluded from further analyzes using the rarefaction filter for environmental heterogeneity available in SDMtoolbox v.2.2 (Brown et al., 2017). The remaining records were managed in two groups: (i) new records and in situ data (model building records); and (ii) existing records (model evaluation records) (ESM 2).
Environmental (predictor) variables were sourced from the bio-oracle database (http://www.bio-oracle.org; Tyberghein et al., 2012; Assis et al., 2018) (Table 2). Bathymetric information was harvested from naturalearthdata.com. These variables were cropped to the study area using the “CropRaster” function of the ENMGadgets R package (Barve & Barve, 2013). Since N. atlantica has few documented occurrence records, we assumed a small number of variables as sufficient (Ficetola et al., 2014). Therefore, a Principal Component Analysis (PCA) was performed to check which variables of the environmental dataset had a higher contribution value (e.g., r > 0.7).
| Variables | Correlations |
|---|---|
| Chlorophyll.max | Primary productivity.max |
| Dissolved oxygen.min | No correlations |
| Nitrate.range | Sillicate.range |
| Primary productivity.max | Chlorophyll.max |
| Salinity.max | Temperature.max, Temperature.range |
| Sillicate.range | Nitrate.range |
| Temperature.max | Salinity.max, Temperature.range |
| Temperature.range | Sillicate.range, Temperature.max |
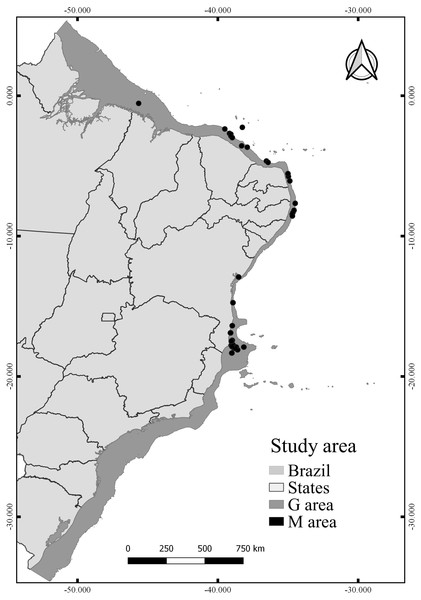
The calibration area for the models (M) corresponds to the entire area to which the species had access at some point (Barve et al., 2011). The choosing of M has been well discussed in the literature (Soberón & Peterson, 2005; Owens et al., 2013), especially to reduce the hypothesis bias regarding the access of species (Anderson & Raza, 2010). Since the known distribution of N. atlantica is limited, we extended the area around the occurrence records by 20 km2 in diameter, thus creating a perimeter (M) representing only the known accessible area for the species (Fig. 1). This method reduces uncertainties about the distribution of the species and the risk of extrapolating the model to new areas beyond the known distribution (Nuñez Penichet et al., 2021; Fulgêncio-Lima et al., 2021; Machado-Stredel, Cobos & Peterson, 2021). Overall, the model projection area (G) extends along the Brazilian Continental shelf (Fig. 1), from the coastline to the shelf-break (0–200 m).
Figure 1: Study area, including the model calibration area (M) and the model projection area (G).
The Maxent software (Phillips, 2005) was used through the dismo package (Hijmans et al., 2013) to model potential habitats (PH) for N. atlantica in the study area, with the Kuenm package (Kükenthal, 1903) applied to find the best parameterization configuration. Candidate models were calibrated using all “feature classes” (FC) combinations available at the Maxent; 29 parameters “Regularization Multiplayer” (RM) (0.1–1 with 0.1 interval and 1–10 with 0,5 intervals), maximum “background” number (100,00), 100 repetitions, and 64 sets of variables.
For each Maxent configuration parameter, two candidate models were created using the “kuenm_val” function, of which one with the complete set of modeling records and the other with 75% of the modeling records. To evaluate candidate models, three criteria were used via “kuenm_ceval” function: (i) models built with 75% of the modeling records were evaluated for statistical significance using the “partial ROC tests” (Peterson, Papeş & Soberón, 2008), and then for performance through the omission rate (using 25% of the modeling records); (ii) models constructed with the complete set of modeling records were evaluated using the corrected small sample Akaike Information Criterion (AICc) (Warren, Glor & Turelli, 2010); and (iii) models that passed in both criteria were projected in G. The best model of those projected in G was chosen according to “partial ROC tests” and omission rates (E = 5%), respectively (Kükenthal, 1903).
The final models were created in G through three types of extrapolations: (1) strict extrapolation, in which the models show a response even if the environmental conditions of G are outside the environmental range of the calibration area (M); (2) without extrapolation, in which areas of G with more extreme environmental conditions than those in the calibration area (M) receive zero response; and (3) extrapolation by fixation, which was the one used to calculate the Potential Suitable Habitat (PH). The final models were built using extrapolation by clamping, where areas of G that are environmentally different from M are fixed on the periphery of the environmental region of the calibration area (M) (Kükenthal, 1903), making the model assign a low environmental suitability value to these areas.
To assess the risk of extrapolating the environmental space from M to G, the multivariate environmental distances between the transfer area (G) and the nearest portion of the calibration region (M) were calculated applying the mobility-oriented parity metric (MOP) (Owens et al., 2013) using the “kuenm_mmop” function. The map indicating the places with a high risk of extrapolation (risk of uncertainty) was constructed using the “kuenm_mop” function. Finally, the binary adequation map was built using maximum threshold values, which are more adequate to species with reduced occurrence records (Liu, White & Newell, 2013). All shapefiles were obtained from open sources (IBGE, http://www.naturalearthdata.com), and the maps were built using the Information System and Geoprocessing software (Quantum GIS (Development Team, 2014)). All data used in the modelling analyzes are available as (Data S1–Data S3).
Results
In situ records
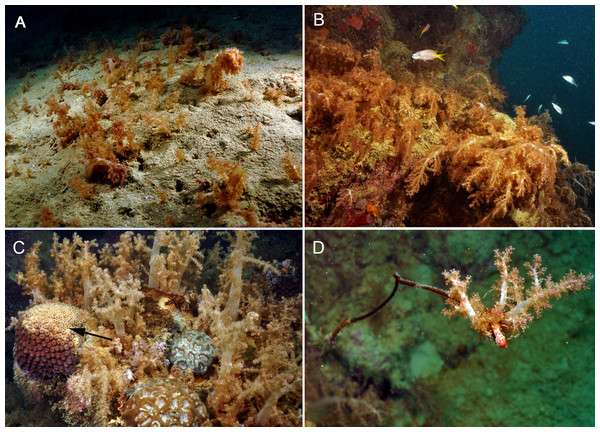
Conventional SCUBA, ROV and technical dive surveys revealed extensive aggregations of N. atlantica dominating deeper soft-substrata (mesophotic depth) on the continental shelf (Fig. 2A; ESM 2, ESM 3) and shallow reef-walls surrounded by sandy bottoms (Figs. 2B–2D). All observed contact interactions were harmful to other coral species (Figs. 2C–2D). Although in situ evidence of OFs was only obtained for the surveyed sites, they may follow the distribution range of N. atlantica. The Cabeço, for example, area with the largest OF found during our surveys had nearly 1 km2 surveyed but the OF limits were not reached.
Figure 2: Substrate dominance and allelopathic competition of Neospongodes atlantica to Brazilian native corals at Timbebas reef (17°28′37.2″S, 39°01′37.2″W, 10–15 m deep).
(A) Aggregation of N. atlantica on sandy bottom in the Abrolhos region, Bahia. (B) N. atlantica colonies dominating a reef edge on Abrolhos reefs. (C) Bleaching and partial necrosis caused by N. atlantica on the scleractinian coral Montastraea cavernosa (arrow). (D) N. atlantica covering a black-coral colony (Stichopathes sp.). All photos by RB Francini-Filho.Morphology
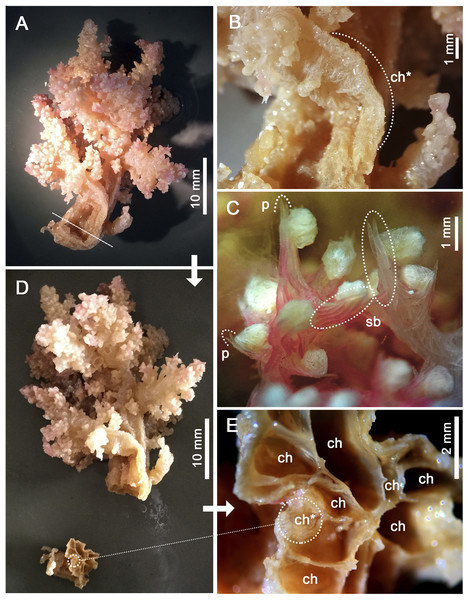
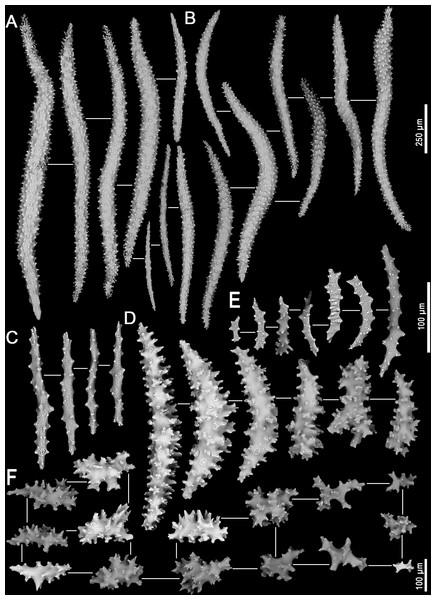
Assessment/identification of museum samples yielded 60 records of Neospongodes atlantica (between ∼1°S and 19°S, from 1 to 75 m depths; Table S2), most of which have not been published before. The species has highly flexible and arborescent colonies rarely taller than 100 mm, with branches standing upwards (Fig. 3A), resulting in a width of about 60 mm. Colonies coloration varies from white, pink to pale brown, and are attached to substrate through slender coenenchyme basal expansions. The polyps occur on the branches, but most are distally concentrated, all with a projecting supporting bundle of sclerites in the form of spindles of up to 1.7 mm in length (Figs. 3C, 4A, Fig. S1). The colony stalk is up to 20 mm wide, have no polyp, and is composed of several longitudinal channels separated by thin walls, in which spindles are common (Figs. 3D, 4D). In most examined samples, one of the channels shows a higher concentration of such sclerites, forming an irregular pseudo-axis (Figs. 3B, 3E). It is important to mention that colony measurements given herein correspond to ethanol-preserved samples, which are mostly contracted. Polyps with an anthocodial armature formed by spindles arranged in chevrons of up to six lines (0.5 to 1.0 mm long), become smaller and more linear towards the tentacles (0.15 to 0.30 mm long) (Figs. 4B, 4C, 4E). Spindles on the supporting bundles often curved and pink-colored (0.3 to 1.2 mm).
Figure 3: Main distinctive morphological characters in Neospongodes atlantica (MOUFPE 431).
(A) Entire colony. (B) View of the stalk with a central channel, in which sclerites occur in a higher density. (C) View of anthocodia on a branch tip showing projecting supporting bundles. (D) Colony with its stalk transversally sectioned, showing longitudinal channels. (E) Colony channels in detail. Abbreviations: ch - channel; p - projecting tip of a spindle; sb - supporting bundle of sclerites; and ch* - pseudo-axis.Figure 4: Sclerites of Neospongodes atlantica Kukenthal, 1903 (MOUFPE 439).
(A) Spindles of the supporting bundle; (B) spindles of the anthocodial points; (C) flattened rods of the crown region; (D) spindles of the interior of the stalk; (E) flattened rods of the adaxial portion of the polyp; (F) sclerites of the surface of the stalk.Molecular and phylogenetic analysis
Molecular analysis resulted in sequences of 662 bp long for the mtMutS, 707 bp for the COI and only 322 bp for the 28S marker. The final alignment, consisting of three concatenated genes (mtMutS, COI, and 28S), included 103 taxa and is 2,181 bp long, of which 1,275 of the 1,840 variable sites are parsimony informative. The best-fit models for the final alignment were the GTR+G (mtMutS), HKY+G (COI), and K80+I+G (28S). Pairwise distance between Stereonephthya cf. cundabiluensis from Palau (GenBank accession numbers: KF915783 and KF955259) and N. atlantica resulted in differences smaller than 0.5% within mitochondrial markers. However, when comparing the 28S dataset, the specimen identified as Dendronephthya sp. from Palau (GenBank accession number: KF915355) is more similar to sequenced N. atlantica (p-distance of 0.094) than to Stereonephthya species, which have a pairwise distance between 0.11 to 0.29.
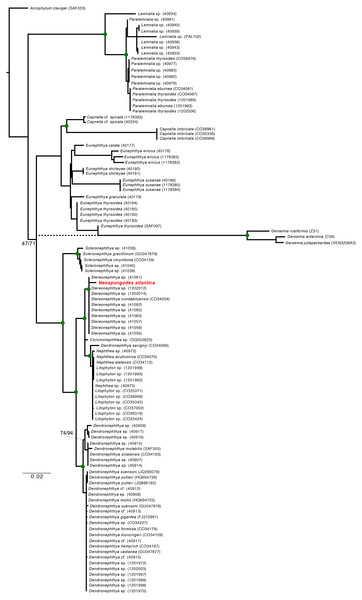
The ML and BI (Fig. 5) phylogenies based on the concatenated alignment unequivocally retrieved a clade containing all Stereonephthya spp. and N. atlantica (95-ML; 100-Posterior Probability [BI]). This clade is close related to that formed by Litophyton, Nephthea, and Dendronephthya savignyi (97-ML; 100-PP). Remaining Dendronephthya representatives tested (30 specimens; 98-ML and 100-PP) were recovered as a sister clade to the aforementioned groups. Most of the remaining genera included in our phylogeny appears to be monophyletic, the exception being Paralemnalia sp. (40981) that is more closely related to Lemnalia spp. than to its congeners. The only discrepancy between ML and BI recovered topologies was the position of the Gersemia clade that although monophyletic, was retrieved diverging from Eunephthya thysoides in the ML and as polytomy in the BI topology (see dashed line in Fig. 5).
Figure 5: Maximum Likelihood and Bayesian inference phylogenetic reconstructions of the family Nephtheidae based on the extended Octocorallia barcoding genes (mtMutS + COI + 28S) concatenated data.
The Brazilian specimen of Neospongodes atlantica sampled from Recife is highlighted in red. Green circles on nodes indicate supports over 80% for ML and posterior probability of 100 (BI). Dashed branch indicate the Gersemia clade leading branch retrieve in BI. Genbank sequences of Nephthea (40972, 40973, CO34070, CO34112) correspond to Litophyton, recently synonymized.Species distribution models
A PCA showed that more than 99% of the environmental variance was explained by groups of two, three, or four variables (Data S2). Pearson correlation selecting eight variables (Data S2), suggested that 64 out of the 93 possible combinations have auto-correlation above 0,8. Thus, we built 24 datasets with two variables, 28 with three variables, and 12 with four (Data S2).
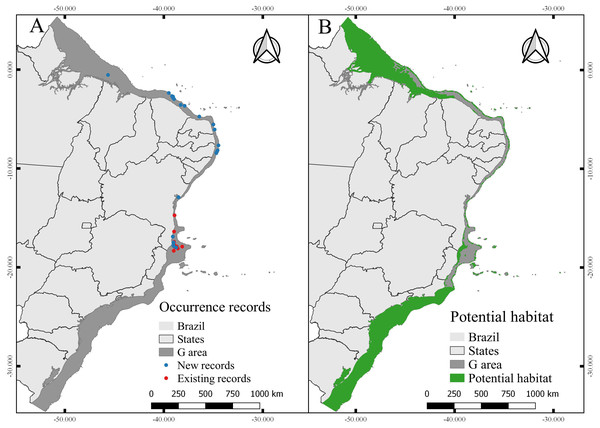
After filtering procedures, a total of 23 records were used for model building (new records) and 12 records were used for model testing (independent records) (Fig. 6). The evaluation of the models calibrated in M resulted in 15 candidates according to omission rates and AICc (Data S3) (Table 3). The final model (from those projected in G) was built using maximum temperature (57.2% of contribution) and maximum silicate (42.8% of contribution), RM = 0.6 and FC = pt.
Figure 6: Occurrence records and current potential habitat.
(A) Modeling records of Neospongodes atlantica (new) and evaluation records (previously existing records). (B) Potential habitat area for N. atlantica in the Brazilian coast, from intertidal zone to shelf-break.| Criterion | Number of models |
|---|---|
| All candidate models | 53,824 |
| Statistically significant models | 24,182 |
| Models that meet the omission rate criteria | 34,193 |
| Models that meet the AICc criteria | 34 |
| Statistically significant models that meet the omission rate criteria | 6,169 |
| Statistically significant models that meet the AICc criteria | 24 |
| Statistically significant models that meet the omission rate and AICc criteria | 15 |
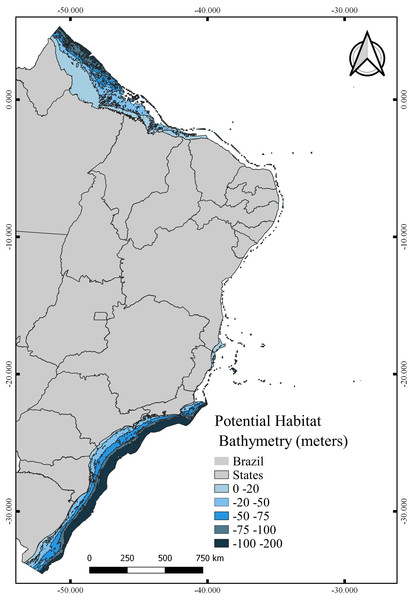
The total area with PH for N. atlantica in the examined area is approximately 553,015.5 km2, occupying 73.07% of the G area (Fig. 6). The use of buffers around the occurrence records to calibrate the model can reduce uncertainties when such model is extrapolated to new areas beyond the calibration area (Ficetola et al., 2014; Fulgêncio-Lima et al., 2021; Machado-Stredel, Cobos & Peterson, 2021). Bathymetric analyzes showed that 29.4% of the PH is between 0 and 20 m deep (162,670 km2), 24.2% from 20 to 50 m (134,382 km2), 16.6% between 50 and 75 m (91,943.9 km2), 10.9% from 75 to 100 m (60,674.6 km2), and 18.6% around 100 and 200 m (103,345 km2) (Fig. 7).
Figure 7: Potential habitat for Neospongodes atlantica at five different depths within the Brazilian coast from the intertidal zone to the end of the continental shelf.
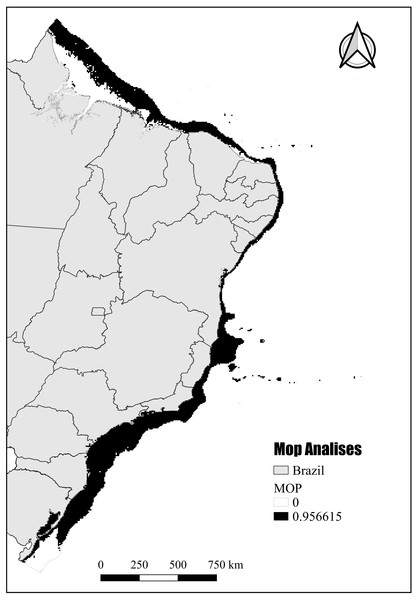
The mobility-oriented parity analysis (MOP) revealed that most of the areas with PH showed a high risk of strict extrapolation within the Brazilian continental shelf (G) (Fig. 8). These G areas have values outside the environmental range of the calibration area (M). However, the regions in the southernmost and northernmost (Amazon reefs) limits showed very low or zero risk of extrapolation (Fig. 8).
Figure 8: Potential habitat end mobility-oriented parity analysis (MOP).
Assessment of the risk of extrapolation of M in G.Discussion
Neospongodes atlantica and the systematics of nephtheids
Nephtheids have been classified mostly based on morphological data. Nonetheless, despite morphology being essential for the correct identification of octocorals, due to plasticity and homoplasy, it is nothing less than challenging to differentiate nephtheid genera and species (Ofwegen & Groenenberg, 2007). Therefore, improved morphological data acquisition techniques, such as sclerite analysis using SEM, combined with genetic data, suggested that, not only Nephtheidae, but many octocoral families and genera were unnatural groupings resulting from poor identifications/descriptions (Verseveldt, 1983; Ofwegen, 2005; Ofwegen & Groenenberg, 2007). For example, Dendronephthya Kükenthal (1905), Litophyton Forskål (1775), and Stereonephthya, genera that were recovered as a well-supported clade in the present study, are morphologically complex and, in general, poorly studied, as discussed in the most recent and comprehensive revisions (Ofwegen & Groenenberg, 2007; Ofwegen, 2020). Although this grouping has received high statistical support, their morphological complexity is reflected in the resulting phylogeny. Exceptions were the genera Capnella, Lemnalia, Paralemnalia, Eunephthya, and Gersemia, in which morphology classifications correspond to well-supported molecular clades (e.g., McFadden et al., 2014).
In their nephtheid molecular analysis, Ofwegen & Groenenberg (2007) found two main clades: (I) Chromonephthea/Stereonephthya; and (II) Nephthea/Litophyton. As a result, Litophyton and Nephthea were later merged (Ofwegen, 2016; Ofwegen, 2020). Although our results corroborate that Nephthya savignyi Ehrenberg (1834), the type species of Dendronephthya, belongs to Litophyton (Ofwegen & Groenenberg, 2007), most of the available Dendronephthya sequences falls as a sister group of their Clades I and II (Fig. 5), indicating that a further revision of Dendronephthya is also needed. Despite several uncertainties regarding the diagnostic morphological characters in nephtheids (Verseveldt, 1983; Ofwegen & Groenenberg, 2007), most of its genera appears to be well-resolved. The recalcitrant taxa are limited to some species in need of additional assessments, such as Neospongodes atlantica, which was recovered herein within a clade composed solely by Stereonephthya representatives (Fig. 5).
Overall, the distinction between Stereonephthya and Neospongodes is based on weak evidence: the presence of an accumulation of sclerites in the center of the stalk of the latter, called herein pseudoaxis (see Verseveldt, 1983; Ofwegen & Groenenberg, 2007). The nature of such an axis was already discussed in the literature and it is known to be convergent in Neospongodes, Scleronephthya, and in several Dendronephthya and Stereonephthya representatives (Verseveldt, 1983). In addition, colony shape is not a good generic diagnostic character (Ofwegen, 2016), as shown by Litophyton savignyi (=Nephthya savignyi). Thus, so far, the polyp armature along with its highly projecting supporting bundle, despite convergent in some genera, seems to be consistent in Stereonephthya and Neospongodes (Fig. 3; Fig. S1). The only uncertainty of such character remains on S. portoricensis, as Bayer (1961) illustrates it with projecting supporting bundles (Fig. S1), a feature reiterated by Verseveldt (1983) although no signs of it is seen in his detailed drawing of the antocodium (Fig. S1). Both Stereonephthya and Neospongodes share well-developed supporting bundles of the polyps with strong projecting tip; ventral side of the polyp stalk has small tiny rods; and strong dorsal points but ventral points poorly supported or lacking (Utinomi, 1954). Thus, based on the current molecular and morphological evidence, there are no reasons to keep both genera separated, as previously suggested by Ofwegen & Groenenberg (2007).
Octocoral forests and their extensive distribution
Our results fill a considerable gap in the distribution of N. atlantica along the Brazilian coast, especially in between the records from Ceará (3°26′S and 38°08′W) and Bahia states (∼12°S to ∼17°S, see Castro, Medeiros & Loiola (2010)). In this area, N. atlantica appears as a minor element in the shallow reef environments, especially in the Abrolhos regions (Laborel, 1969; Castro et al., 2005; Leão & Kikuchi, 2000). However, it forms dense aggregations on soft bottoms at mesophotic depths (Moura et al., 2013). These results contrast with the Indo-Pacific region, where the majority of shallow water nephtheids are associated with zooxanthellae (Litophyton, Lemnalia, Capnella, etc.) (Fabricius & Alderslade, 2001; Schubert, Brown & Rossi, 2017), and only a few such depth generalists are found below 25–30 m. Nonetheless, the symbiotic taxa of this family are also found in mesophotic depths, on both hard and soft substrates (e.g., Umbellulifera). Eastern nephteids may form either monospecific carpets or diverse aggregations with as many as 31 species per 600 m2 (Bayer, 1961; Tursch & Tursch, 1982) and, in reef communities, they may total a biomass 10 times higher than that of scleractinian corals (Benayahu & Loya, 1987; Tursch & Tursch, 1982).
Despite being considered a typical reef species, the mesophotic OFs found in Brazil suggest that N. atlantica should be referred to as a soft-bottom species, as its occurrence in reefs is restricted to reef-sand interfaces and reef-walls influenced by the amount of suitable habitat (soft bottom) around them, as recorded for the Cabeço and Timbebas Reef (Fig. 2). Such a behavior is shared by other nephtheid genera such as Dendronephthya and Umbellulifera, both considered typical sand dwellers (Fabricius & Alderslade, 2001).
Although inhabiting the photic zone, N. atlantica is an azooxanthellate species that, similarly to the Indo-Pacific Dendronephthya and Stereonephthya (see Fabricius & McCorry, 2006; Ofwegen & Groenenberg, 2007), appears to have a preference for low-light exposition. Whereas light availability limits the occurrence of the majority of Indo-Pacific zooxanthellate soft-corals to shallow and well-lit depths Tursch & Tursch, 1982), N. atlantica forms aggregations to up to 95 m deep (Pérez, Neves & Oliveira, 2011). In terms of substrate, based on data available from museum samples and the Abrolhos stations from Moura et al. (2013), N. atlantica appears to have a preference for sandy bottoms with high CaCO3 and moderate silt/mud concentrations. Given its success in such substrata, reef communities close to sediment discharges (e.g., dredging or river discharges) should be carefully monitored. In that context, most areas modeled as PSH for N. atlantica were found at the northernmost and southernmost limits of the Brazilian Continental Shelf, where no record of the species are yet known but great quantities of sand, silt, and mud dischargers occur from the Amazon and La Plata rivers respectively. Although these unknown occurrence areas might be considered as an accurate representation of the spatial extent that provides habitable conditions for the targeted species (Peterson, Papeş & Soberón, 2008), the MOP analysis indicated that most of the PH in those areas point to the risk of high extrapolation. Such areas have values outside the climatic range of the calibration area (see Raghavan et al., 2019) and, therefore, caution is required when assessing the probability of N. atlantica occurrence in these areas.
Taking into consideration that (i) morphological differentiation between Neospongodes and Stereonephthya (if it really exists) is yet to be proposed, (ii) N. atlantica is molecularly identical to Indo-Pacific Stereonephthya, and (iii) N. atlantica is the only representative of the family in the Southwestern Atlantic (Chromonephthea braziliensis has been reported as non-indigenous (NIS) in Brazil (see Ofwegen (2005))), we consider it inappropriate to keep both genera separated. Therefore, according to the International Code of Zoological Nomenclature, all species names currently associated with Stereonephthya should be moved to Neospongodes, as the latter has date priority in relation to the former. Unfortunately, the data presented herein suggest that N. atlantica was probably introduced in the Southwestern Atlantic as earlier as 1903 (i.e., the year of its description) either by Portuguese or Dutch vessels transiting within the marine trade routes (Ebert, 2003; see also Morandini et al., 2017). Otherwise, the alternative hypothesis would be a common origin shared with Stereonephthya portoricensis, a species found in mesophotic to deep Caribbean waters (Cairns, 2017) in a previous Atlantic-Pacific-split scenario. To test these hypotheses, it would be necessary to sequence S. portoricensis, which is unavailable at this moment.
Considering the hypothesis that N. atlantica is a NIS is alarming, even though it would not be the first long-hidden introduced species into Brazilian waters. For example, the once thought native octocoral Carijoa riisei and the bivalve Perna perna have been suggested to be introduced a long time ago (Grigg, 2003; Concepción et al., 2010; Pierri, Fossari & Magalhães, 2016; Galván-Villa & Ríos-Jara, 2018; Lima et al., 2018), even though there is not a consensus in the case of the bivalve (Calazans et al., 2021). Some of the problems of NIS are related to the fact that they can become invasive, altering the ecosystem function and, therefore, causing biodiversity loss and economic impacts (Bax et al., 2003; Chakraborty, 2019; Carpinelli et al., 2020). In the case of N. atlantica, its populations have apparently increased recently on soft (as previous expeditions did not report it) and hard-substrate. Nonetheless, as most of the records presented herein are from mesophotic depths (see Table S2), its spread along the Brazilian continental shelf remained mostly unnoticed. In our field observations, it was found to cause tissue necrosis in one of the main reef-building scleractinian species in Brazil, the great-star-coral Montastraea cavernosa (Fig. 2), a species that is abundant at mesophotic depths (Francini-Filho et al., 2019). Many soft corals, including nephtheids, bear diverse secondary metabolites, which makes them unpalatable, as well as strong spatial competitors (Tursch, 1982; Sammarco, La Barre & Coll, 1987; Allam et al., 2021). Terpenoids either in tissue or in the surrounding water make these soft-corals superior competitors even without direct contact with other organisms (allelopathy), such as scleractinian corals (Coll et al., 1982; Fabricius, 1997). For example, an invasive xeniid octocoral has invaded the Venezuelan Caribbean and, more recently, its abundance has surpassed that of all other benthic taxa in that region (Ruiz-Allais, Benayahu & Lasso-Alcalá, 2021). Invasive xeniids and other non-indigenous octocoral species from other families have also been reported in Brazil (Mantelatto et al., 2018; Carpinelli et al., 2020; Menezes et al., 2021), raising concerns on the fate of invaded localities biodiversity and, consequently, ecosystem function. Therefore, we recommend that populations of N. atlantica should be monitored in order to keep track of its spread and eventual invasiveness in Brazilian marine environments, along with other recently aquarium-introduced octocorals (Mantelatto et al., 2018; Carpinelli et al., 2020).