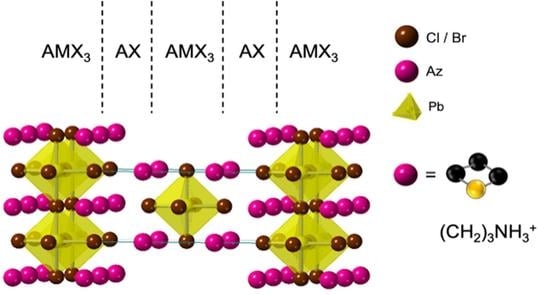
Azetidinium Lead Halide Ruddlesden–Popper Phases
Abstract
:1. Introduction
2. Method
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lichtenberg, F.; Herrnberger, A.; Wiedenmann, K. Synthesis, structural, magnetic and transport properties of layered perovskite-related titanates, niobates and tantalates of the type AnBnO3n+2, A′Ak−1BkO3k+1 and AmBm−1O3m. Prog. Solid State Chem. 2008, 36, 253–387. [Google Scholar] [CrossRef]
- Aleksandrov, K.S.; Beznosikov, V.V. Hierarchies of perovskite-like crystals (Review). Phys. Solid State 1997, 39, 695–715. [Google Scholar] [CrossRef]
- Balz, D.; Plieth, K. Die Struktur des Kaliumnickelfluorids, K2NiF. Z. Elektrochem. Ber. Bunsenges. Phys. Chem. 1955, 59, 545–551. [Google Scholar] [CrossRef]
- Ruddlesden, S.N.; Popper, P. New compounds of the K2NiF4 type. Acta Crystallogr. 1957, 10, 538–539. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Cao, D.H.; Clark, D.J.; Young, J.; Rondinelli, J.M.; Jang, J.I.; Hupp, J.T.; Kanatzidis, M.G. Ruddlesden-Popper hybrid lead iodide perovskite 2D homologous semiconductors. Chem. Mater. 2016, 28, 2852–2867. [Google Scholar] [CrossRef]
- Du, K.Z.; Tu, Q.; Zhang, X.; Han, Q.; Liu, J.; Zauscher, S.; Mitzi, D.B. Two-dimensional lead(II) halide-based hybrid perovskites templated by acene alkylamines: Crystal structures, optical properties, and piezoelectricity. Inorg. Chem. 2017, 56, 9291–9302. [Google Scholar] [CrossRef]
- Mitzi, D.B. A layered solution crystal growth technique and the crystal structure of (C6H5C2H4NH3)2PbCl4. J. Solid State Chem. 1999, 145, 694–704. [Google Scholar] [CrossRef]
- Spanopoulos, I.; Hadar, I.; Ke, W.; Tu, Q.; Chen, M.; Tsai, H.; He, Y.; Shekhawat, G.; Dravid, V.P.; Wasielewski, M.R.; et al. Uniaxial expansion of the 2D Ruddlesden–Popper perovskite family for improved environmental stability. J. Am. Chem. Soc. 2019, 141, 5518–5534. [Google Scholar] [CrossRef]
- Tsai, H.; Nie, W.; Blancon, J.C.; Stoumpos, C.C.; Asadpour, R.; Harutyunyan, B.; Neukirch, A.J.; Verduzco, R.; Crochet, J.J.; Tretiak, S.; et al. High-efficiency two-dimensional Ruddlesden–Popper perovskite solar cells. Nature 2016, 536, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Leng, K.; Abdelwahab, I.; Verzhbitskiy, I.; Telychko, M.; Chu, L.; Fu, W.; Chi, X.; Guo, N.; Chen, Z.; Chen, Z.; et al. Molecularly thin two-dimensional hybrid perovskites with tunable optoelectronic properties due to reversible surface relaxation. Nat. Mater. 2018, 17, 908–914. [Google Scholar] [CrossRef]
- Ren, H.; Yu, S.; Chao, L.; Xia, Y.; Sun, Y.; Zuo, S.; Li, F.; Niu, T.; Yang, Y.; Ju, H.; et al. Efficient and stable Ruddlesden–Popper perovskite solar cell with tailored interlayer molecular interaction. Nat. Photonics 2020, 14, 154–163. [Google Scholar] [CrossRef]
- Tian, J.; Cordes, D.B.; Slawin, A.M.Z.; Zysman-Colman, E.; Morrison, F.D. Progressive polytypism and bandgap tuning in azetidinium lead halide perovskites. Inorg. Chem. 2021, 60, 12247–12254. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zysman-Colman, E.; Morrison, F.D. Compositional variation in hybrid organic-inorganic lead halide perovskites: Kinetically versus thermodynamically controlled synthesis. Chem. Mater. 2021, 33, 3650–3659. [Google Scholar] [CrossRef]
- Kieslich, G.; Sun, S.; Cheetham, A.K.; Cheetham, T.; Gregor, K.; Shijing, S.; Anthony, K.C. Solid-state principles applied to organic-inorganic perovskites: New tricks for an old dog. Chem. Sci. 2014, 5, 4712–4715. [Google Scholar] [CrossRef]
- Levchuk, I.; Osvet, A.; Tang, X.; Brandl, M.; Perea, J.D.; Hoegl, F.; Matt, G.J.; Hock, R.; Batentschuk, M.; Brabec, C.J. Brightly luminescent and color-tunable formamidinium lead halide perovskite FAPbX3 (X = Cl, Br, I) colloidal nanocrystals. Nano Lett. 2017, 17, 2765–2770. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Tian, J.; Cai, Z.; Peng, L.; Yang, L.; Wei, D. Perovskite heterojunction based on CH3NH3PbBr3 single crystal for high-sensitive self-powered photodetector. Appl. Phys. Lett. 2016, 109, 233303. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Cordes, D.B.; Quarti, C.; Beljonne, D.; Slawin, A.M.Z.; Zysman-Colman, E.; Morrison, F.D. Stable 6H organic-inorganic hybrid lead perovskite and competitive formation of 6H and 3C perovskite structure with mixed A cations. ACS Appl. Energy Mater. 2019, 2, 5427–5437. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System (GSAS); Los Alamos National Laboratory: Carlsbad, NM, USA, 2004; pp. 86–748. [Google Scholar]
- Yu, J.C.; Kim, D.B.; Jung, E.D.; Lee, B.R.; Song, M.H. High-performance perovskite light-emitting diodes via morphological control of perovskite films. Nanoscale 2016, 8, 7036–7042. [Google Scholar] [CrossRef] [Green Version]
- Ganguli, D. Cationic radius ratio and formation of K2NiF4-type compounds. J. Solid State Chem. 1979, 30, 353–356. [Google Scholar] [CrossRef]
- Panetta, R.; Righini, G.; Colapietro, M.; Barba, L.; Tedeschi, D.; Polimeni, A.; Ciccioli, A.; Latini, A. Azetidinium lead iodide: Synthesis, structural and physico-chemical characterization. J. Mater. Chem. A 2018, 6, 10135–10148. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Pal, P.; Saha, S.; Banik, A.; Sarkar, A.; Biswas, K. All-solid-state mechanochemical synthesis and post-synthetic transformation of inorganic perovskite-type halides. Chem. Eur. J. 2018, 24, 1811–1815. [Google Scholar] [CrossRef] [PubMed]
- Saski, M.; Prochowicz, D.; Marynowski, W.; Lewiński, J. Mechanosynthesis, optical, and morphological properties of MA, FA, CsSnX3 (X = I, Br) and phase-pure mixed-halide MASnIxBr3–x perovskites. Eur. J. Inorg. Chem. 2019, 2019, 2680–2684. [Google Scholar] [CrossRef]
- Lee, S.; Levi, R.D.; Qu, W.; Lee, S.C.; Randall, C.A. Band-gap nonlinearity in perovskite structured solid solutions. J. Appl. Phys. 2010, 107, 023523. [Google Scholar] [CrossRef]
- Chatterjee, S.; Payne, J.; Irvine, J.T.S.; Pal, A.J. Bandgap bowing in a zero-dimensional hybrid halide perovskite derivative: Spin-orbit coupling: Versus lattice strain. J. Mater. Chem. A 2020, 8, 4416–4427. [Google Scholar] [CrossRef]
- Noh, J.H.; Im, S.H.; Heo, J.H.; Mandal, T.N.; Seok, S. Il Chemical management for colorful, efficient, and stable inorganic-organic hybrid nanostructured solar cells. Nano Lett. 2013, 13, 1764–1769. [Google Scholar] [CrossRef]
- Wang, W.; Su, J.; Zhang, L.; Lei, Y.; Wang, D.; Lu, D.; Bai, Y. Growth of mixed-halide perovskite single crystals. CrystEngComm 2018, 20, 1635–1643. [Google Scholar] [CrossRef]
- Hu, Z.; Lin, Z.; Su, J.; Zhang, J.; Chang, J.; Hao, Y. A review on energy band-gap engineering for perovskite photovoltaics. Sol. RRL 2019, 3, 1900304. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.; Zysman-Colman, E.; Morrison, F.D. Azetidinium Lead Halide Ruddlesden–Popper Phases. Molecules 2021, 26, 6474. https://doi.org/10.3390/molecules26216474
Tian J, Zysman-Colman E, Morrison FD. Azetidinium Lead Halide Ruddlesden–Popper Phases. Molecules. 2021; 26(21):6474. https://doi.org/10.3390/molecules26216474
Chicago/Turabian StyleTian, Jiyu, Eli Zysman-Colman, and Finlay D. Morrison. 2021. "Azetidinium Lead Halide Ruddlesden–Popper Phases" Molecules 26, no. 21: 6474. https://doi.org/10.3390/molecules26216474