Not All That Glitters Is Gold: Barcoding Effort Reveals Taxonomic Incongruences in Iconic Ross Sea Sea Stars
Abstract
:1. Introduction
2. Materials and Methods
- -
- 2006/08.01 (“The coastal ecosystem of Terra Nova Bay” in the Latitudinal Gradient Program—LGP) (“XXV” expedition, 2009/2010).
- -
- 2010/A1.10 (BAMBi; Barcoding of Antarctic Marine Biodiversity) (“XXVII” expedition, 2011/2012 and (“XXVIII” expedition, 2012/2013).
- -
- 2009/A1.09 (Diversità genetica spazio temporale di endoparassiti delle regioni polari: uno studio per la valutazione dell’impatto dei cambiamenti globali sulle reti trofiche marine) (“XXVIII” expedition, 2012/2013).
2.1. Sampling and DNA Extraction
2.2. Species Delimitation Methods
2.3. Molecular Data Gathering
2.4. Literature Review
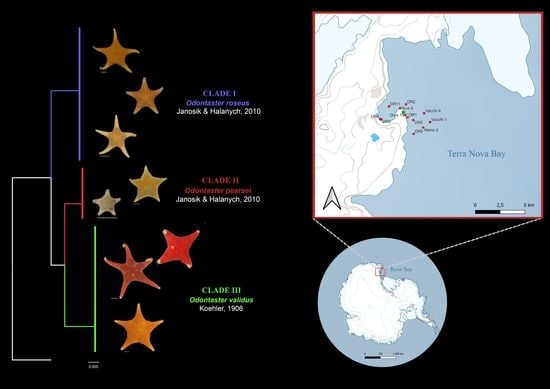
3. Results
3.1. Species Delimitation Methods
3.2. Sequences Database Review
3.3. Morphological Analysis
3.4. Scientific Literature Revision of Odontaster in the Ross Sea Quadrant
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mah, C.L.; Blake, D.B. Global Diversity and Phylogeny of the Asteroidea (Echinodermata). PLoS ONE 2012, 7, e35644. [Google Scholar] [CrossRef] [Green Version]
- Mah, C.; Linse, K.; Copley, J.; Marsh, L.; Rogers, A.; Clague, D.; Foltz, D. Description of a New Family, New Genus, and Two New Species of Deep-Sea Forcipulatacea (Asteroidea), Including the First Known Sea Star from Hydrothermal Vent Habitats. Zool. J. Linn. Soc. 2015, 174, 93–113. [Google Scholar] [CrossRef] [Green Version]
- Dilman, A.B.; Minin, K.V.; Petrov, N.B. New Record of the Wood-Associated Sea Star Caymanostella, with Notes on the Phylogenetic Position of the Family Caymanostellidae (Asteroidea). Zool. J. Linn. Soc. 2022, 194, 14–35. [Google Scholar] [CrossRef]
- Goharimanesh, M.; Ghassemzadeh, F.; De Kegel, B.; Van Hoorebeke, L.; Stöhr, S.; Mirshamsi, O.; Adriaens, D. The Evolutionary Relationship between Arm Vertebrae Shape and Ecological Lifestyle in Brittle Stars (Echinodermata: Ophiuroidea). J. Anat. 2021, 240, 1034–1047. [Google Scholar] [CrossRef]
- Stöhr, S.; O’Hara, T.D.; Thuy, B. Global Diversity of Brittle Stars (Echinodermata: Ophiuroidea). PLoS ONE 2012, 7, e31940. [Google Scholar] [CrossRef] [PubMed]
- Goharimanesh, M.; Stohr, S.; Mirshamsi, O.; Ghassemzadeh, F.; Adriaens, D. Interactive Identification Key to All Brittle Star Families (Echinodermata; Ophiuroidea) Leads to Revised Morphological Descriptions. Eur. J. Taxon. 2021, 766, 1–63. [Google Scholar] [CrossRef]
- Moreau, C. Diversity and Phylogeography of Southern Ocean Sea Stars (Asteroidea). PhD Thesis, Université Bourgogne Franche-Comté, Besancon, France, Université libre de Bruxelles, Bruxelles, Belgium, 2019. [Google Scholar]
- De Broyer, C.; Clarke, A.; Koubbi, P.; Pakhomov, E.; Scott, F.; Vanden Berghe, E.; Danis, B. Register of Antarctic Marine Species. 2017. Available online: http://www.marinespecies.org/rams (accessed on 17 February 2022).
- Jamieson, A. The Hadal Zone: Life in the Deepest Oceans; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Danis, B.; Griffiths, H.J.; Jangoux, M. Asteroidea. In Biogeographic Atlas of the Southern Ocean; Scientific Committee on Antarctic Research: San Francisco, CA, USA, 2014. [Google Scholar]
- Moreau, C.V.; Aguera, A.; Jossart, Q.; Danis, B. Southern Ocean Asteroidea: A Proposed Update for the Register of Antarctic Marine Species. Biodivers. Data J. 2015, 3, e7062. [Google Scholar] [CrossRef]
- Moreau, C.; Mah, C.; Agüera, A.; Améziane, N.; Barnes, D.; Crokaert, G.; Eléaume, M.; Griffiths, H.; Guillaumot, C.; Hemery, L.G. Antarctic and Sub-Antarctic Asteroidea Database. ZooKeys 2018, 747, 141–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearse, J.S. Slow Developing Demersal Embryos and Larvae of the Antarctic Sea Star Odontaster Validus. Mar. Biol. 1969, 3, 110–116. [Google Scholar] [CrossRef]
- Stanwell-Smith, D.; Peck, L.S. Temperature and Embryonic Development in Relation to Spawning and Field Occurrence of Larvae of Three Antarctic Echinoderms. Biol. Bull. 1998, 194, 44–52. [Google Scholar] [CrossRef]
- Pearse, J.S.; Mooi, R.; Lockhart, S.J.; Brandt, A. Brooding and Species Diversity in the Southern Ocean: Selection for Brooders or Speciation within Brooding Clades? In Smithsonian at the Poles: Contributions to International Polar Year Science; Smithsonian Institution: Washington, DC, USA, 2009. [Google Scholar]
- Chown, S.L.; Clarke, A.; Fraser, C.I.; Cary, S.C.; Moon, K.L.; McGeoch, M.A. The Changing Form of Antarctic Biodiversity. Nature 2015, 522, 431–438. [Google Scholar] [CrossRef]
- Williams, S.T. Species Boundaries in the Starfish Genus Linckia. Mar. Biol. 2000, 136, 137–148. [Google Scholar] [CrossRef]
- Zulliger, D.E.; Lessios, H.A. Phylogenetic Relationships in the Genus Astropecten Gray (Paxillosida: Astropectinidae) on a Global Scale: Molecular Evidence for Morphological Convergence, Species-Complexes and Possible Cryptic Speciation. Zootaxa 2010, 2504, 14. [Google Scholar] [CrossRef]
- Xiao, N.; Liu, R.; Yuan, S.; Sha, Z. A Preliminary Phylogenetic Analysis of Luidia (Paxillosida: Luidiidae) from Chinese Waters with Cytochrome Oxidase Subunit I (COI) Sequences. J. Ocean. Univ. China 2013, 12, 459–468. [Google Scholar] [CrossRef]
- Hopkins, G.W.; Freckleton, R.P. Declines in the Numbers of Amateur and Professional Taxonomists: Implications for Conservation. In Animal Conservation; Cambridge University Press: Cambridge, UK, 2002; Volume 5, pp. 245–249. [Google Scholar]
- Knott, K.E.; Wray, G.A. Controversy and Consensus in Asteroid Systematics: New Insights to Ordinal and Familial Relationships. Am. Zool. 2000, 40, 382–392. [Google Scholar] [CrossRef] [Green Version]
- Janies, D.A.; Voight, J.R.; Daly, M. Echinoderm Phylogeny Including Xyloplax, a Progenetic Asteroid. Syst. Biol. 2011, 60, 420–438. [Google Scholar] [CrossRef]
- Mah, C.; Foltz, D. Molecular Phylogeny of the Valvatacea (Asteroidea: Echinodermata). Zool. J. Linn. Soc. 2011, 161, 769–788. [Google Scholar] [CrossRef] [Green Version]
- Linchangco, G.V., Jr.; Foltz, D.W.; Reid, R.; Williams, J.; Nodzak, C.; Kerr, A.M.; Miller, A.K.; Hunter, R.; Wilson, N.G.; Nielsen, W.J. The Phylogeny of Extant Starfish (Asteroidea: Echinodermata) Including Xyloplax, Based on Comparative Transcriptomics. Mol. Phylogenetics Evol. 2017, 115, 161–170. [Google Scholar] [CrossRef] [Green Version]
- Moreau, C.; Danis, B.; Jossart, Q.; Eléaume, M.; Sands, C.; Achaz, G.; Agüera, A.; Saucède, T. Is Reproductive Strategy a Key Factor in Understanding the Evolutionary History of Southern Ocean Asteroidea (Echinodermata)? Ecol. Evol. 2019, 9, 8465–8478. [Google Scholar] [CrossRef] [Green Version]
- Moreau, C.; Jossart, Q.; Danis, B.; Eléaume, M.; Christiansen, H.; Guillaumot, C.; Downey, R.; Saucède, T. The High Diversity of Southern Ocean Sea Stars (Asteroidea) Reveals Original Evolutionary Pathways. Prog. Oceanogr. 2021, 190, 102472. [Google Scholar] [CrossRef]
- Sands, C.J.; O’Hara, T.D.; Martín-Ledo, R. Pragmatic Assignment of Species Groups Based on Primary Species Hypotheses: The Case of a Dominant Component of the Southern Ocean Benthic Fauna. Front. Mar. Sci. 2021, 8, 1371. [Google Scholar] [CrossRef]
- Dell, R.K. Antarctic Benthos. In Advances in Marine Biology; Elsevier: Amsterdam, The Netherlands, 1972; Volume 10, pp. 1–216. [Google Scholar]
- Clarke, A.; Crame, J.A. The Origin of the Southern Ocean Marine Fauna. Geol. Soc. Lond. Spec. Publ. 1989, 47, 253–268. [Google Scholar] [CrossRef]
- Griffiths, H.J.; Barnes, D.K.; Linse, K. Towards a Generalized Biogeography of the Southern Ocean Benthos. J. Biogeogr. 2009, 36, 162–177. [Google Scholar] [CrossRef]
- Hebert, P.D.; Cywinska, A.; Ball, S.L.; DeWaard, J.R. Biological Identifications through DNA Barcodes. Proc. R. Soc. London Ser. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, Automatic Barcode Gap Discovery for Primary Species Delimitation. Mol. Ecol. 2012, 21, 1864–1877. [Google Scholar] [CrossRef]
- Pearse, J.S.; Bosch, I. Photoperiodic Regulation of Gametogenesis in the Antarctic Sea Star Odontaster Validus Koehler: Evidence for a Circannual Rhythm Modulated by Light. Invertebr. Reprod. Dev. 2002, 41, 73–81. [Google Scholar] [CrossRef]
- Janosik, A.M.; Halanych, K.M. Unrecognized Antarctic Biodiversity: A Case Study of the Genus Odontaster (Odontasteridae; Asteroidea). Integr. Comp. Biol. 2010, 50, 981–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janosik, A.M.; Mahon, A.R.; Halanych, K.M. Evolutionary History of Southern Ocean Odontaster Sea Star Species (Odontasteridae; Asteroidea). Polar Biol. 2011, 34, 575–586. [Google Scholar] [CrossRef]
- Agüera, A.; Collard, M.; Jossart, Q.; Moreau, C.; Danis, B. Parameter Estimations of Dynamic Energy Budget (DEB) Model over the Life History of a Key Antarctic Species: The Antarctic Sea Star Odontaster Validus Koehler, 1906. PLoS ONE 2015, 10, e0140078. [Google Scholar] [CrossRef] [Green Version]
- Janosik, A.M. Seeing Stars: A Molecular and Morphological Investigation of the Odontasteridae (Asteroidea). Ph.D. Thesis, Auburn University, Auburn, AL, USA, 2012. [Google Scholar]
- Dayton, P.K. Toward an Understanding of Community Resilience and the Potential Effects of Enrichments to the Benthos at McMurdo Sound, Antarctica. In Proceedings of the Colloquium on Conservation Problems in Antarctica, Blacksberg, VA, USA, 10–12 September 1972; pp. 81–96. [Google Scholar]
- McClintock, J.B.; Angus, R.A.; Ho, C.P.; Amsler, C.D.; Baker, B.J. Intraspecific Agonistic Arm-Fencing Behavior in the Antarctic Keystone Sea Star Odontaster Validus Influences Prey Acquisition. Mar. Ecol. Prog. Ser. 2008, 371, 297–300. [Google Scholar] [CrossRef]
- Kim, S.L.; Thurber, A.; Hammerstrom, K.; Conlan, K. Seastar Response to Organic Enrichment in an Oligotrophic Polar Habitat. J. Exp. Mar. Biol. Ecol. 2007, 346, 66–75. [Google Scholar] [CrossRef]
- Peirano, A.; Bordone, A.; Marini, S.; Piazza, P.; Schiaparelli, S. A Simple Time-Lapse Apparatus for Monitoring Macrozoobenthos Activity in Antarctica. Antarct. Sci. 2016, 28, 473–474. [Google Scholar] [CrossRef]
- Dell’Acqua, O.; Ferrando, S.; Chiantore, M.; Asnaghi, V. The Impact of Ocean Acidification on the Gonads of Three Key Antarctic Benthic Macroinvertebrates. Aquat. Toxicol. 2019, 210, 19–29. [Google Scholar] [CrossRef]
- Signa, G.; Calizza, E.; Costantini, M.L.; Tramati, C.; Caputi, S.S.; Mazzola, A.; Rossi, L.; Vizzini, S. Horizontal and Vertical Food Web Structure Drives Trace Element Trophic Transfer in Terra Nova Bay, Antarctica. Environ. Pollut. 2019, 246, 772–781. [Google Scholar] [CrossRef]
- Peck, L.S.; Webb, K.E.; Miller, A.; Clark, M.S.; Hill, T. Temperature Limits to Activity, Feeding and Metabolism in the Antarctic Starfish Odontaster Validus. Mar. Ecol. Prog. Ser. 2008, 358, 181–189. [Google Scholar] [CrossRef]
- Gonzalez-Bernat, M.J.; Lamare, M.; Barker, M. Effects of Reduced Seawater PH on Fertilisation, Embryogenesis and Larval Development in the Antarctic Seastar Odontaster Validus. Polar Biol. 2013, 36, 235–247. [Google Scholar] [CrossRef]
- Held, C. Molecular Evidence for Cryptic Speciation within the Widespread Antarctic Crustacean Ceratoserolis Trilobitoides (Crustacea, Isopoda). In Antarctic Biology in a Global Context; Cambridge University Press: Cambridge, UK, 2003; pp. 135–139. [Google Scholar]
- Held, C.; Wägele, J.W. Cryptic Speciation in the Giant Antarctic Isopod Glyptonotus Antarcticus (Isopoda, Valvifera, Chaetiliidae). Sci. Mar. 2005, 69, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Ceballos, S.G.; Roesti, M.; Matschiner, M.; Fernández, D.A.; Damerau, M.; Hanel, R.; Salzburger, W. Phylogenomics of an Extra-Antarctic Notothenioid Radiation Reveals a Previously Unrecognized Lineage and Diffuse Species Boundaries. BMC Evol. Biol. 2019, 19, 1–14. [Google Scholar] [CrossRef]
- Moles, J.; Berning, M.I.; Hooker, Y.; Padula, V.; Wilson, N.G.; Schrödl, M. Due South: The Evolutionary History of Sub-Antarctic and Antarctic Tritoniidae Nudibranchs. Mol. Phylogenetics Evol. 2021, 162, 107209. [Google Scholar] [CrossRef]
- Bickford, D.; Lohman, D.J.; Sodhi, N.S.; Peter, K.L.; Ng, P.K.L.; Rudolf, M.; Eier, R.; Winker, K. Ingram KK, Das I. Cryptic species as a window on diversity and conservation. Trends Ecol. Evol. 2007, 22, 148–155. [Google Scholar] [CrossRef]
- Brasier, M.J.; Wiklund, H.; Neal, L.; Jeffreys, R.; Linse, K.; Ruhl, H.; Glover, A.G. DNA Barcoding Uncovers Cryptic Diversity in 50% of Deep-Sea Antarctic Polychaetes. R. Soc. Open Sci. 2016, 3, 160432. [Google Scholar] [CrossRef] [Green Version]
- Arrigo, K.R.; van Dijken, G.L.; Bushinsky, S. Primary Production in the Southern Ocean, 1997–2006. J. Geophys. Res. Ocean. 2008, 113, C08004. [Google Scholar] [CrossRef]
- Cerrano, C.; Bavestrello, G.; Calcinai, B.; Cattaneo-Vietti, R.; Sarà, A. Asteroids Eating Sponges from Tethys Bay, East Antarctica. Antarct. Sci. 2000, 12, 425–426. [Google Scholar] [CrossRef]
- Chiantore, M.; Cattaneo-Vietti, R.; Elia, L.; Guidetti, M.; Antonini, M. Reproduction and Condition of the Scallop Adamussium Colbecki (Smith 1902), the Sea-Urchin Sterechinus Neumayeri (Meissner 1900) and the Sea-Star Odontaster Validus Koehler 1911 at Terra Nova Bay (Ross Sea): Different Strategies Related to Inter-Annual Variations in Food Availability. Polar Biol. 2002, 25, 251–255. [Google Scholar]
- Chiantore, M.; Guidetti, M.; Cavallero, M.; De Domenico, F.; Albertelli, G.; Cattaneo-Vietti, R. Sea Urchins, Sea Stars and Brittle Stars from Terra Nova Bay (Ross Sea, Antarctica). Polar Biol. 2006, 29, 467–475. [Google Scholar] [CrossRef]
- Clark, H.E.S. The Fauna of the Ross Sea, Part 3, Asteroidea. N. Z. Depart. Sci. Indus. Res. Bull. 151 N. Z. Oceanogr. Inst. Mem. 1963, 21, 1–84. [Google Scholar]
- Bosch, I.; Pearse, J.S. Developmental Types of Shallow-Water Asteroids of McMurdo Sound, Antarctica. Mar. Biol. 1990, 104, 41–46. [Google Scholar] [CrossRef]
- Moore, M.; Manahan, D.T. Variation among Females in Egg Lipid Content and Developmental Success of Echinoderms from McMurdo Sound, Antarctica. Polar Biol. 2007, 30, 1245–1252. [Google Scholar] [CrossRef]
- C-CAMLR-XXXV, Report of the thirty-fifth meeting of the Scientific Committee, Hobart, Australia, 17–21 October, Annex 6, 3.2, 3.7-3.9. 2016. CCAMLR CONSERVATION MEASURE 91-05 (2016) for the Ross Sea Region Marine Protected Area, Specifically, Addressing the Priorities of Annex 91-05/C. 2016. Available online: https://www.ccamlr.org/en/system/files/e-sc-xxxv.pdf (accessed on 21 March 2022).
- Faranda, F.M.; Guglielmo, L.; Ianora, A. The Italian Oceanographic Cruises in the Ross Sea (1987–95): Strategy, General Considerations and Description of the Sampling Sites. In Ross Sea Ecology; Springer: Berlin/Heidelberg, Germany, 2000; pp. 1–13. [Google Scholar]
- Kurtz, D.D.; Bromwich, D.H. Satellite Observed Behavior of the Terra Nova Bay Polynya. J. Geophys. Res. Ocean. 1983, 88, 9717–9722. [Google Scholar] [CrossRef] [Green Version]
- Mangoni, O.; Saggiomo, M.; Bolinesi, F.; Castellano, M.; Povero, P.; Saggiomo, V.; DiTullio, G.R. Phaeocystis Antarctica Unusual Summer Bloom in Stratified Antarctic Coastal Waters (Terra Nova Bay, Ross Sea). Mar. Environ. Res. 2019, 151, 104733. [Google Scholar] [CrossRef]
- Jansen, J.; Hill, N.A.; Dunstan, P.K.; Eléaume, M.P.; Johnson, C.R. Taxonomic Resolution, Functional Traits, and the Influence of Species Groupings on Mapping Antarctic Seafloor Biodiversity. Front. Ecol. Evol. 2018, 6, 81. [Google Scholar] [CrossRef] [Green Version]
- Fisher, W.K. Asteroidea. Discov. Rep. 1940, 20, 69–306. [Google Scholar]
- Corstorphine, E.A. DNA Barcoding of Echinoderms: Species Diversity and Patterns of Molecular Evolution. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 2010. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA Primers for Amplification of Mitochondrial Cytochrome c Oxidase Subunit I from Diverse Metazoan Invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Leray, M.; Knowlton, N. Censusing Marine Eukaryotic Diversity in the Twenty-First Century. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150331. [Google Scholar] [CrossRef]
- Blake, D.B. A Classification and Phylogeny of Post-Palaeozoic Sea Stars (Asteroidea: Echinodermata). J. Nat. Hist. 1987, 21, 481–528. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Tamura, K. Estimation of the Number of Nucleotide Substitutions When There Are Strong Transition-Transversion and G+ C-Content Biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian Phylogenetic Inference under Mixed Models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [Green Version]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian Inference of Phylogenetic Trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [Green Version]
- Erixon, P.; Svennblad, B.; Britton, T.; Oxelman, B. Reliability of Bayesian Posterior Probabilities and Bootstrap Frequencies in Phylogenetics. Syst. Biol. 2003, 52, 665–673. [Google Scholar] [CrossRef]
- Hebert, P.D.; Ratnasingham, S.; De Waard, J.R. Barcoding Animal Life: Cytochrome c Oxidase Subunit 1 Divergences among Closely Related Species. Proc. R. Soc. London Ser. B Biol. Sci. 2003, 270, S96–S99. [Google Scholar] [CrossRef] [Green Version]
- Meyer, C.P.; Paulay, G. DNA Barcoding: Error Rates Based on Comprehensive Sampling. PLoS Biol. 2005, 3, e422. [Google Scholar] [CrossRef] [Green Version]
- Ratnasingham, S.; Hebert, P.D. A DNA-Based Registry for All Animal Species: The Barcode Index Number (BIN) System. PLoS ONE 2013, 8, e66213. [Google Scholar] [CrossRef] [Green Version]
- Fujisawa, T.; Barraclough, T.G. Delimiting Species Using Single-Locus Data and the Generalized Mixed Yule Coalescent Approach: A Revised Method and Evaluation on Simulated Data Sets. Syst. Biol. 2013, 62, 707–724. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Kapli, P.; Pavlidis, P.; Stamatakis, A. A General Species Delimitation Method with Applications to Phylogenetic Placements. Bioinformatics 2013, 29, 2869–2876. [Google Scholar] [CrossRef] [Green Version]
- Fujita, M.K.; Leaché, A.D.; Burbrink, F.T.; McGuire, J.A.; Moritz, C. Coalescent-Based Species Delimitation in an Integrative Taxonomy. Trends Ecol. Evol. 2012, 27, 480–488. [Google Scholar] [CrossRef]
- Leavitt, S.D.; Moreau, C.S.; Thorsten Lumbsch, H. The Dynamic Discipline of Species Delimitation: Progress toward Effectively Recognizing Species Boundaries in Natural Populations. In Recent Advances in Lichenology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 11–44. [Google Scholar]
- Heimeier, D.; Lavery, S.; Sewell, M.A. Using DNA Barcoding and Phylogenetics to Identify Antarctic Invertebrate Larvae: Lessons from a Large Scale Study. Mar. Genom. 2010, 3, 165–177. [Google Scholar] [CrossRef]
- Smith, W.O., Jr.; Sedwick, P.N.; Arrigo, K.R.; Ainley, D.G.; Orsi, A.H. The Ross Sea in a Sea of Change. Oceanography 2012, 25, 90–103. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.H.; Kim, S.; Choi, S.K.; Moon, K.; Choi, H.-G.; Ko, Y.W.; Hawes, I.; Kim, S.-H.; Kim, J.H.; Park, S.R. Composition and Structure of the Marine Benthic Community in Terra Nova Bay, Antarctica: Responses of the Benthic Assemblage to Disturbances. PLoS ONE 2019, 14, e0225551. [Google Scholar] [CrossRef] [Green Version]
- Rossi, L.; Sporta Caputi, S.; Calizza, E.; Careddu, G.; Oliverio, M.; Schiaparelli, S.; Costantini, M.L. Antarctic Food Web Architecture under Varying Dynamics of Sea Ice Cover. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Caputi, S.S.; Careddu, G.; Calizza, E.; Fiorentino, F.; Maccapan, D.; Rossi, L.; Costantini, M.L. Seasonal Food Web Dynamics in the Antarctic Benthos of Tethys Bay (Ross Sea): Implications for Biodiversity Persistence under Different Seasonal Sea-Ice Coverage. Front. Mar. Sci. 2020, 7, 1046. [Google Scholar] [CrossRef]
- Brueggeman, P. Underwater Field Guide to Ross Island & McMurdo Sound, Antarctica; The National Science Foundation’s Office of Polar Progams: San Diego, CA, USA, 1998.
- Rauschert, M.; Arntz, W. Antarctic Macrobenthos: A Field Guide of the Invertebrates Living at the Antarctic Seafloor; Arntz & Rauschert Selbstverlag: Bremen, Germany, 2015. [Google Scholar]
- Peck, L.S.; Clark, M.S.; Dunn, N.I. Morphological Variation in Taxonomic Characters of the Antarctic Starfish Odontaster Validus. Polar Biol. 2018, 41, 2159–2165. [Google Scholar] [CrossRef]
- Ghiglione, C.; Alvaro, M.C.; Griffiths, H.J.; Linse, K.; Schiaparelli, S. Ross Sea Mollusca from the Latitudinal Gradient Program: R/V Italica 2004 Rauschert Dredge Samples. ZooKeys 2013, 341, 37–48. [Google Scholar]
- Ghiglione, C.; Alvaro, M.C.; Cecchetto, M.; Canese, S.; Downey, R.; Guzzi, A.; Mazzoli, C.; Piazza, P.; Rapp, H.T.; Sarà, A. Porifera Collection of the Italian National Antarctic Museum (MNA), with an Updated Checklist from Terra Nova Bay (Ross Sea). ZooKeys 2018, 758, 137–156. [Google Scholar] [CrossRef] [Green Version]
- Piazza, P.; Blażewicz-Paszkowycz, M.; Ghiglione, C.; Alvaro, M.C.; Schnabel, K.; Schiaparelli, S. Distributional Records of Ross Sea (Antarctica) Tanaidacea from Museum Samples Stored in the Collections of the Italian National Antarctic Museum (MNA) and the New Zealand National Institute of Water and Atmospheric Research (NIWA). ZooKeys 2014, 451, 49–60. [Google Scholar]
- Selbmann, L.; Onofri, S.; Zucconi, L.; Isola, D.; Rottigni, M.; Ghiglione, C.; Piazza, P.; Alvaro, M.C.; Schiaparelli, S. Distributional Records of Antarctic Fungi Based on Strains Preserved in the Culture Collection of Fungi from Extreme Environments (CCFEE) Mycological Section Associated with the Italian National Antarctic Museum (MNA). MycoKeys 2015, 10, 57. [Google Scholar]
- Cecchetto, M.; Alvaro, M.C.; Ghiglione, C.; Guzzi, A.; Mazzoli, C.; Piazza, P.; Schiaparelli, S. Distributional Records of Antarctic and Sub-Antarctic Ophiuroidea from Samples Curated at the Italian National Antarctic Museum (MNA): Check-List Update of the Group in the Terra Nova Bay Area (Ross Sea) and Launch of the MNA 3D Model ‘Virtual Gallery’. ZooKeys 2017, 705, 61–79. [Google Scholar] [CrossRef] [Green Version]
- Cecchetto, M.; Lombardi, C.; Canese, S.; Cocito, S.; Kuklinski, P.; Mazzoli, C.; Schiaparelli, S. The Bryozoa Collection of the Italian National Antarctic Museum, with an Updated Checklist from Terra Nova Bay, Ross Sea. ZooKeys 2019, 1, 812. [Google Scholar] [CrossRef] [Green Version]
- Garlasché, G.; Karimullah, K.; Iakovenko, N.; Velasco-Castrillón, A.; Janko, K.; Guidetti, R.; Rebecchi, L.; Cecchetto, M.; Schiaparelli, S.; Jersabek, C.D. A Data Set on the Distribution of Rotifera in Antarctica. Biogeogr./Ital. Biogeogr. Soc. 2020, 35, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Bonello, G.; Grillo, M.; Cecchetto, M.; Giallain, M.; Granata, A.; Guglielmo, L.; Pane, L.; Schiaparelli, S. Distributional Records of Ross Sea (Antarctica) Planktic Copepoda from Bibliographic Data and Samples Curated at the Italian National Antarctic Museum (MNA): Checklist of Species Collected in the Ross Sea Sector from 1987 to 1995. ZooKeys 2020, 969, 1. [Google Scholar] [CrossRef]








| Expedition | Station | Location | Year | Latitude | Longitude | Depth (m) | Sample Vouchers | N |
|---|---|---|---|---|---|---|---|---|
| PNRA XXV Exp 09/10 | Dive 9 | Tethys Bay “zecca” | 2009 | −74.9027 | 164.10255 | 23 | MNA-02814 | 1 |
| Dive 19 | Road Bay | 2010 | −74.69647 | 164.12007 | 15 | MNA-02902 | 1 | |
| PNRA XXVII Exp 11/12 | DR1 | Road Bay | 2012 | −74.69848 | 164.12812 | 100 | MNA-03430, 04282, 04283 | 3 |
| DR3 | Tethys Bay | 2012 | −74.70005 | 164.03873 | 60 | MNA-03582 | 1 | |
| DR4 | Tethys Bay | 2012 | −74.70010 | 164.03502 | 198 | MNA-04276 | 1 | |
| DR9 | Faraglione | 2012 | −74.71337 | 164.14903 | 150 | MNA-03791, 03812, 03825, 03832, 03841, 08034,08035, 08036 | 8 | |
| PNRA XXVIII Exp 12/13 | DR5 | Road Bay | 2013 | −74.70087 | 164.14793 | 150 | MNA-05817, 08037, 08038, 08039 | 4 |
| DR9 | MZS (‘fossa’) | 2013 | −74.68090 | 164.21433 | 522 | MNA-06116 | 1 | |
| DR11 | Tethys Bay | 2013 | −74.68872 | 164.06493 | 222 | MNA-06486, 06489, 06490 | 3 | |
| Vacchi 1 | Tethys Bay | 2013 | −74.70262 | 164.20502 | 569 | MNA-05430 | 1 | |
| Vacchi 4 | Tethys Bay | 2013 | −74.69478 | 164.18458 | 454 | MNA-06331, 08021, 08022, 08023, 08024, 08025, 08026, 08027, 08028, 08029, 08030, 08031, 08032, 08033 | 14 | |
| PNRA XXIX Exp 13/14 | DR2 | “Dorsale” MZS | 2014 | −74.68677 | 164.12278 | 94 | MNA-08043 | 1 |
| Mario 3 | Punta Stocchino | 2014 | −74.70750 | 164.18167 | 281 | MNA-08042 | 1 |
| Region | Direction | Primer | Sequence (5’-3’) | Reference |
|---|---|---|---|---|
| COI | F | LCOech1aF1 | TTTTTTCTACTAAACACAAGGATATTGG | Corstorphine, 2010 [65] |
| LCO1490 | GGTCAACAAATCATAAAGATATTGG | Folmer et al., 1994 [66] | ||
| R | HCO2198 | TAAACTTCAGGGTGACCAAAAAATCA | Folmer et al., 1994 [66] |
| BOLD BIN | Species | n | Sample Vouchers |
|---|---|---|---|
| AAE2388 | Odontaster roseus Janosik & Halanych, 2010 | 17 | MNA-02814, 03791, 03812, 03832, 03841, 05817, 06486, 06490, 08024, 08025, 08027, 08028, 08033, 08036, 08037, 08038, 08043 |
| AAK3286 | Odontaster validus Koehler, 1906 | 16 | MNA-02902, 03430, 03582, 03825, 04276, 04282, 04283, 05430, 06331, 08021, 08022, 08026, 08029, 08030, 08031, 08035 |
| AAO2072 | Odontaster pearsei Janosik & Halanych, 2010 | 7 | MNA-06116, 06489, 08023, 08032, 08034, 08039, 08042 |
| Sample ID | Sequence Code BOLD | Sequence Code GenBank | Mined from | Wrong ID | Correct ID | Year | Location | BOLD BIN | Published |
|---|---|---|---|---|---|---|---|---|---|
| 36438 | NZEC742-09 | BOLD | O. meridionalis | O. roseus I | 2008 | Ross Sea | BOLD:AAE2388 | ||
| 38186 | NZEC743-09 | BOLD | O. meridionalis | O. roseus II | 2008 | Out Ross | BOLD:AAE2389 | ||
| 38512-1 | NZEC744-09 | BOLD | O. meridionalis | O. roseus II | 2008 | Out Ross | BOLD:AAE2389 | ||
| 38719-1 | NZEC745-09 | BOLD | O. meridionalis | O. roseus II | 2008 | Out Ross | BOLD:AAE2389 | ||
| 38719-2 | NZEC746-09 | BOLD | O. meridionalis | O. roseus II | 2008 | Out Ross | BOLD:AAE2389 | ||
| A02.15T | GBMIN874-12 | GU227088.1 | GenBank | O. meridionalis | O. roseus I | 2002 | McMurdo Sound | BOLD:AAE2388 | Heimeier et al., 2010 |
| A04N.08 | GBMIN878-12 | GU227092.1 | GenBank | O. validus | 2004 | Cape Hallett | BOLD:AAK3286 | Heimeier et al., 2010 | |
| As 68 | GQ294374.1 | GenBank | O. validus | 2011 | Ross Sea | Janosick et al., 2011 | |||
| As 86 | GQ294384.1 | GenBank | O. validus | 2011 | Ross Sea | Janosick et al., 2011 | |||
| As 87 | GQ294385.1 | GenBank | O. validus | 2011 | Ross Sea | Janosick et al., 2011 | |||
| As 88 | GQ294386.1 | GenBank | O. validus | 2011 | Ross Sea | Janosick et al., 2011 | |||
| As 33, 34, 69, 70, 71,72 | O. validus | 2011 | Ross Sea | Janosick et al., 2011 | |||||
| MNA-3582 | TCTNB082-15 | MK811555 | GenBank | O. validus | 2019 | Terra Nova Bay | BOLD:AAK3286 | Rossi et al., 2019 | |
| MNA-4276 | TCTNB079-15 | MK811610 | GenBank | O. validus | 2019 | Terra Nova Bay | BOLD:AAK3286 | Rossi et al., 2019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzzi, A.; Alvaro, M.C.; Danis, B.; Moreau, C.; Schiaparelli, S. Not All That Glitters Is Gold: Barcoding Effort Reveals Taxonomic Incongruences in Iconic Ross Sea Sea Stars. Diversity 2022, 14, 457. https://doi.org/10.3390/d14060457
Guzzi A, Alvaro MC, Danis B, Moreau C, Schiaparelli S. Not All That Glitters Is Gold: Barcoding Effort Reveals Taxonomic Incongruences in Iconic Ross Sea Sea Stars. Diversity. 2022; 14(6):457. https://doi.org/10.3390/d14060457
Chicago/Turabian StyleGuzzi, Alice, Maria Chiara Alvaro, Bruno Danis, Camille Moreau, and Stefano Schiaparelli. 2022. "Not All That Glitters Is Gold: Barcoding Effort Reveals Taxonomic Incongruences in Iconic Ross Sea Sea Stars" Diversity 14, no. 6: 457. https://doi.org/10.3390/d14060457