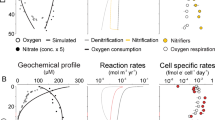
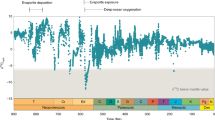
Abstract
Microorganisms that transform and oxidize organic material (that is, heterotrophs) play a fundamental role in the geochemical cycling of key elements in the ocean. Through their growth and activity, heterotrophic microorganisms degrade much of the organic matter produced by phytoplankton in the surface ocean, leading to the regeneration and redistribution of nutrients and carbon back into the water column. However, most organic matter is physically too large to be taken up directly by heterotrophic microorganisms. Consequently, many heterotrophs secrete exoenzymes that break down large molecules outside the cell into smaller substrates that can then be directly taken up by the cell. The complex nature of the biochemical systems that microorganisms use to secrete these enzymes suggests that they were unlikely to have been present in the earliest heterotrophs. In a pre-exoenzyme ocean, heterotrophic microorganisms would only be able to access a small fraction of organic matter such that most dead phytoplankton biomass would have passed directly through the water column and settled onto the seafloor. Here we synthesize existing geobiological evidence to examine the fate of organic matter in the absence of exoenzymes in early oceans. We propose that on an Earth before exoenzymes, organic matter preservation, metal availability and phosphorus recycling would have operated differently than they do on the contemporary Earth.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Neidhardt, F. C. & Umbarger, E. H. Chemical Composition of Escherichia Coli 2nd edn (American Society of Microbiology, 1996).
Benz, R. & Bauer, K. Permeation of hydrophilic molecules through the outer membrane of gram‐negativ bacteria: review of bacterial porins. Eur. J. Biochem. 176, 1–19 (1988).
Arnosti, C. Microbial extracellular enzymes and the marine carbon cycle. Annu. Rev. Mar. Sci. 3, 401–425 (2011).
Mahmoudi, N., Hagen, S. M., Hazen, T. C. & Steen, A. D. Patterns in extracellular enzyme activity and microbial diversity in deep-sea Mediterranean sediments. Deep Sea Res. Pt I 158, 103231 (2020).
Schmidt, J. M., Royalty, T. M., Lloyd, K. G. & Steen, A. D. Potential activities and long lifetimes of organic carbon-degrading extracellular enzymes in deep subsurface sediments of the Baltic Sea. Front. Microbiol. 12, 702015 (2021).
Datta, M. S., Sliwerska, E., Gore, J., Polz, M. F. & Cordero, O. X. Microbial interactions lead to rapid micro-scale successions on model marine particles. Nat. Commun. 7, 119651 (2016).
Mahmoudi, N., Enke, T. N., Beaupré, S. R., Teske, A. P., Cordero, O. X. & Pearson, A. Illuminating microbial species-specific effects on organic matter remineralization in marine sediments. Environ. Microbiol. 22, 1734–1747 (2020).
Burdige, D. J. Preservation of organic matter in marine sediments: controls, mechanisms, and an imbalance in sediment organic carbon budgets? Chem. Rev. 107, 467–485 (2007).
Green, E. R. & Mecsas, J. Bacterial secretion systems: an overview. Microbiol. Spectr. 4, 4.1 (2016). 13.
Dodd, M. S. et al. Evidence for early life in Earth’s oldest hydrothermal vent precipitates. Nature 543, 60–64 (2017).
Gruen, D. S., Wolfe, J. M. & Fournier, G. P. Paleozoic diversification of terrestrial chitin-degrading bacterial lineages. BMC Evol. Biol. 19, 34 (2019).
Godfrey, L. V. & Falkowski, P. G. The cycling and redox state of nitrogen in the Archaean ocean. Nat. Geosci. 2, 725–729 (2009).
Czaja, A. D., Johnson, C. M., Beard, B. L., Roden, E. E., Li, W. & Moorbath, S. Biological Fe oxidation controlled deposition of banded iron formation in the ca. 3770 Ma Isua Supracrustal Belt (West Greenland). Earth Planet. Sci. Lett. 363, 192–203 (2013).
Warke, M. R., Strauss, H. & Schröder, S. Positive cerium anomalies imply pre-GOE redox stratification and manganese oxidation in Paleoproterozoic shallow marine environments. Precambr. Res. 344, 105767 (2020).
Grossman, A. S., Mauer, T. J., Forest, K. T. & Goodrich-Blair, H. A widespread bacterial secretion system with diverse substrates. mBio 12, e01956–01921 (2021).
Meuskens, I., Saragliadis, A., Leo, J. C. & Linke, D. Type v secretion systems: an overview of passenger domain functions. Front. Microbiol. 10, 1163 (2019).
Korotkov, K. V., Sandkvist, M. & Hol, W. G. J. The type II secretion system: biogenesis, molecular architecture and mechanism. Nat. Rev. Microbiol. 10, 336–351 (2012).
Vetter, Y., Deming, J., Jumars, P. A. & Krieger-Brockett, B. A predictive model of bacterial foraging by means of freely released extracellular enzymes. Microb. Ecol. 36, 75–92 (1998).
Ebrahimi, A., Schwartzman, J. & Cordero, O. X. Cooperation and spatial self-organization determine rate and efficiency of particulate organic matter degradation in marine bacteria. Proc. Natl Acad. Sci. USA 116, 23309–23316 (2019).
Reintjes, G., Arnosti, C., Fuchs, B. & Amann, R. Selfish, sharing and scavenging bacteria in the Atlantic Ocean: a biogeographical study of bacterial substrate utilisation. ISME J. 13, 1119–1132 (2019).
Krupke, A., Hmelo, L. R., Ossolinski, J. E., Mincer, T. J. & Van Mooy, B. A. Quorum sensing plays a complex role in regulating the enzyme hydrolysis activity of microbes associated with sinking particles in the ocean. Front. Mar. Sci. 3, 55 (2016).
Cuskin, F., Lowe, E. C., Temple, M. J., Zhu, Y., Cameron, E. A. & Pudlo, N. A. et al. Human gut Bacteroidetes can utilize yeast mannan through a selfish mechanism. Nature 517, 165–169 (2015).
Moran, M. A. & Zepp, R. G. Role of photoreactions in the formation of biologically labile compounds from dissolved organic matter. Limnol. Oceanogr. 42, 1307–1316 (2003).
Baltar, F., Arístegui, J., Gasol, J. M., Sintes, E., van Aken, H. M. & Herndl, G. J. High dissolved extracellular enzymatic activity in the deep central Atlantic Ocean. Aquat. Microb. Ecol. 58, 287–302 (2010).
Quigg, A. et al. From nano-gels to marine snow: a synthesis of gel formation processes and modeling efforts involved with particle flux in the ocean. Gels 7, 114 (2021).
Kipp, M. A., Krissansen-Totton J. & Catling D. C. High organic burial efficiency is required to explain mass balance in Earth’s early carbon cycle. Glob. Biogeochem. Cycles 35, e2020GB006707 (2021).
Buick, R., Des Marais, D. J. & Knoll, A. H. Stable isotopic compositions of carbonates from the Mesoproterozoic Bangemall Group, northwestern Australia. Chem. Geol. 123, 153–171 (1995).
Kipp, M. A. & Stüeken, E. E. Biomass recycling and Earth’s early phosphorus cycle. Sci. Adv. 3, eaao4795 (2017).
Moore, E. K., Jelen, B. I., Giovannelli, D., Raanan, H. & Falkowski, P. G. Metal availability and the expanding network of microbial metabolisms in the Archaean eon. Nat. Geosci. 10, 629–636 (2017).
Bruland, K. W. & Franks, R. P. in Trace Metals in Sea Water (eds. Goldberg, E.D. et al.) 395–414 (Springer, 1983).
Anbar, A. D. & Knoll, A. H. Proterozoic ocean chemistry and evolution: a bioinorganic bridge? Science 297, 1137–1142 (2002).
Morel, F. M. & Price, N. M. The biogeochemical cycles of trace metals in the oceans. Science 300, 944–947 (2003).
Noble, A. E., Ohnemus, D. C., Hawco, N. J., Lam, P. J. & Saito, M. A. Coastal sources, sinks and strong organic complexation of dissolved cobalt within the US North Atlantic GEOTRACES transect GA03. Biogeosciences 14, 2715–2739 (2017).
Toner, B. M. et al. Preservation of iron(ii) by carbon-rich matrices in a hydrothermal plume. Nat. Geosci. 2, 197–201 (2009).
Robbins, L. et al. Authigenic iron oxide proxies for marine zinc over geological time and implications for eukaryotic metallome evolution. Geobiology 11, 295–306 (2013).
Benner, R. Loose ligands and available iron in the ocean. Proc. Natl Acad. Sci. USA 108, 893–894 (2011).
Swaren, L., Alessi, D. S., Owttrim, G. W. & Konhauser, K. O. Acid-base properties of Synechococcus-derived organic matter. Geochim. Cosmochim. Acta 315, 89–100 (2021).
Zhang, J., Kattner, G. & Koch, B. P. Interactions of trace elements and organic ligands in seawater and implications for quantifying biogeochemical dynamics: a review. Earth Sci. Rev. 192, 631–649 (2019).
Reinhard, C. T. et al. Evolution of the global phosphorus cycle. Nature 541, 386–389 (2017).
Kipp, M. A. A double-edged sword: the role of sulfate in anoxic marine phosphorus cycling through Earth history. Geophys. Res. Lett. 49, e2022GL099817 (2022).
Lomas, M. W. et al. Sargasso Sea phosphorus biogeochemistry: an important role for dissolved organic phosphorus (DOP). Biogeosciences 7, 695–710 (2010).
Hori T., Horiguchi M. & Hayashi A. Biochemistry of Natural CP compounds (Maruzen Ltd, 1984).
Luo, H., Benner, R., Long, R. A. & Hu, J. Subcellular localization of marine bacterial alkaline phosphatases. Proc. Natl Acad. Sci. USA 106, 21219–21223 (2009).
Ingall, E. D., Bustin, R. & Van Cappellen, P. Influence of water column anoxia on the burial and preservation of carbon and phosphorus in marine shales. Geochim. Cosmochim. Acta 57, 303–316 (1993).
Van Cappellen, P. & Ingall, E. D. Benthic phosphorus regeneration, net primary production, and ocean anoxia: a model of the coupled marine biogeochemical cycles of carbon and phosphorus. Paleoceanography 9, 677–692 (1994).
Canfield, D. E. A new model for Proterozoic ocean chemistry. Nature 396, 450–453 (1998).
Alcott, L. J., Mills, B. J., Bekker, A. & Poulton, S. W. Earth’s Great Oxidation Event facilitated by the rise of sedimentary phosphorus recycling. Nat. Geosci. 15, 210–215 (2022).
Garcia, A. K. & Kaçar, B. How to resurrect ancestral proteins as proxies for ancient biogeochemistry. Free Radic. Biol. Med. 140, 260–269 (2019).
Zimmerman, A. E., Martiny, A. C. & Allison, S. D. Microdiversity of extracellular enzyme genes among sequenced prokaryotic genomes. ISME J. 7, 1187–1199 (2013).
Fakhraee, M., Tarhan, L. G., Planavsky, N. J. & Reinhard, C. T. A largely invariant marine dissolved organic carbon reservoir across Earth’s history. Proc. Natl Acad. Sci. USA 118, e2103511118 (2021).
Lyons, T. W., Diamond, C. W., Planavsky, N. J., Reinhard, C. T. & Li, C. Oxygenation, life, and the planetary system during Earth’s middle history: an overview. Astrobiology 21, 906–923 (2021).
Acknowledgements
This research was supported financially by Natural Sciences & Engineering Research Council of Canada (NSERC) grants to K.O.K. (RGPIN-2020-05189) and G.P.H. N.M. was supported by a New Frontiers in Research Fund Exploration Grant (NFRFE-2019-00794). A.D.S. was supported by NSF grant numbers OPP-2147046 and OCE- 2145434. We thank A. Grossman for helpful conversations about enzyme secretion systems.
Author information
Authors and Affiliations
Contributions
N.M. and A.D.S conceptualized the Perspective. All authors extensively contributed ideas and provided critical feedback in the research, interpretation and writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Geoscience thanks Jason Sylvan, Joanne Boden and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: James Super, in collaboration with the Nature Geoscience team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mahmoudi, N., Steen, A.D., Halverson, G.P. et al. Biogeochemistry of Earth before exoenzymes. Nat. Geosci. 16, 845–850 (2023). https://doi.org/10.1038/s41561-023-01266-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41561-023-01266-4