Abstract
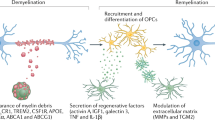
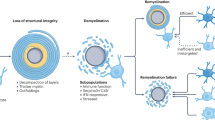
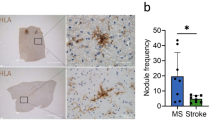
As resident macrophages of the CNS, microglia are critical immune effectors of inflammatory lesions and associated neural dysfunctions. In multiple sclerosis (MS) and its animal models, chronic microglial inflammatory activity damages myelin and disrupts axonal and synaptic activity. In contrast to these detrimental effects, the potent phagocytic and tissue-remodelling capabilities of microglia support critical endogenous repair mechanisms. Although these opposing capabilities have long been appreciated, a precise understanding of their underlying molecular effectors is only beginning to emerge. Here, we review recent advances in our understanding of the roles of microglia in animal models of MS and demyelinating lesions and the mechanisms that underlie their damaging and repairing activities. We also discuss how the structured organization and regulation of the genome enables complex transcriptional heterogeneity within the microglial cell population at demyelinating lesions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Reich, D. S., Lucchinetti, C. F. & Calabresi, P. A. Multiple sclerosis. N. Engl. J. Med. 378, 169–180 (2018).
Gold, S. M., Willing, A., Leypoldt, F., Paul, F. & Friese, M. A. Sex differences in autoimmune disorders of the central nervous system. Semin. Immunopathol. 41, 177–188 (2019).
Walton, C. et al. Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS, third edition. Mult. Scler. 26, 1816–1821 (2020).
Lassmann, H. The contribution of neuropathology to multiple sclerosis research. Eur. J. Neurol. 29, 2869–2877 (2022).
Luchetti, S. et al. Progressive multiple sclerosis patients show substantial lesion activity that correlates with clinical disease severity and sex: a retrospective autopsy cohort analysis. Acta Neuropathol. 135, 511–528 (2018).
Lucchinetti, C. et al. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann. Neurol. 47, 707–717 (2000).
Chitnis, T. & Prat, A. A roadmap to precision medicine for multiple sclerosis. Mult. Scler. 26, 522–532 (2020).
Prineas, J. W., Barnard, R. O., Kwon, E. E., Sharer, L. R. & Cho, E. S. Multiple sclerosis: remyelination of nascent lesions. Ann. Neurol. 33, 137–151 (1993).
Patrikios, P. et al. Remyelination is extensive in a subset of multiple sclerosis patients. Brain 129, 3165–3172 (2006).
Yong, V. W. Microglia in multiple sclerosis: protectors turn destroyers. Neuron 110, 3534–3548 (2022).
Steinman, L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell 85, 299–302 (1996).
Absinta, M., Lassmann, H. & Trapp, B. D. Mechanisms underlying progression in multiple sclerosis. Curr. Opin. Neurol. 33, 277–285 (2020).
Healy, L. M., Stratton, J. A., Kuhlmann, T. & Antel, J. The role of glial cells in multiple sclerosis disease progression. Nat. Rev. Neurol. 18, 237–248 (2022).
Airas, L. & Yong, V. W. Microglia in multiple sclerosis—pathogenesis and imaging. Curr. Opin. Neurol. 35, 299–306 (2022).
Cree, B. A. C., Oksenberg, J. R. & Hauser, S. L. Multiple sclerosis: two decades of progress. Lancet Neurol. 21, 211–214 (2022).
Rivest, S. Regulation of innate immune responses in the brain. Nat. Rev. Immunol. 9, 429–439 (2009).
Ransohoff, R. M. & Perry, V. H. Microglial physiology: unique stimuli, specialized responses. Annu. Rev. Immunol. 27, 119–145 (2009).
Tremblay, M. E. et al. The role of microglia in the healthy brain. J. Neurosci. 31, 16064–16069 (2011).
O’Loughlin, E., Madore, C., Lassmann, H. & Butovsky, O. Microglial phenotypes and functions in multiple sclerosis. Cold Spring Harb. Perspect. Med. 8, a028993 (2018).
Schirmer, L., Schafer, D. P., Bartels, T., Rowitch, D. H. & Calabresi, P. A. Diversity and function of glial cell types in multiple sclerosis. Trends Immunol. 42, 228–247 (2021).
Singh, S. et al. Microglial nodules in early multiple sclerosis white matter are associated with degenerating axons. Acta Neuropathol. 125, 595–608 (2013).
van Horssen, J. et al. Clusters of activated microglia in normal-appearing white matter show signs of innate immune activation. J. Neuroinflammation 9, 156 (2012).
van den Bosch, A. M. R. et al. Ultrastructural axon-myelin unit alterations in MS correlate with inflammation. Ann. Neurol. 93, 856–870 (2022).
Kuhlmann, T. et al. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 133, 13–24 (2017).
Zrzavy, T. et al. Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain 140, 1900–1913 (2017).
Ramaglia, V. et al. Multiplexed imaging of immune cells in staged multiple sclerosis lesions by mass cytometry. eLife 8, e48051 (2019).
Patani, R., Balaratnam, M., Vora, A. & Reynolds, R. Remyelination can be extensive in multiple sclerosis despite a long disease course. Neuropathol. Appl. Neurobiol. 33, 277–287 (2007).
Park, C. et al. The landscape of myeloid and astrocyte phenotypes in acute multiple sclerosis lesions. Acta Neuropathol. Commun. 7, 130 (2019).
Masuda, T. et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566, 388–392 (2019). This work provides the earliest, most comprehensive characterization of gene signatures at the single-cell level of microglia that populate myelin lesions in individuals with MS.
Schirmer, L. et al. Neuronal vulnerability and multilineage diversity in multiple sclerosis. Nature 573, 75–82 (2019).
Absinta, M. et al. A lymphocyte–microglia–astrocyte axis in chronic active multiple sclerosis. Nature 597, 709–714 (2021). This study investigates, at deep resolution, the different cell types and their states of activity at edges of chronic active lesions in MS; data suggest that microglia endowed with iron-processing capabilities are closely intertwined with these lesions.
Bottcher, C. et al. Single-cell mass cytometry reveals complex myeloid cell composition in active lesions of progressive multiple sclerosis. Acta Neuropathol. Commun. 8, 136 (2020).
Jakel, S. et al. Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature 566, 543–547 (2019).
Miedema, A. et al. Brain macrophages acquire distinct transcriptomes in multiple sclerosis lesions and normal appearing white matter. Acta Neuropathol. Commun. 10, 8 (2022).
Menassa, D. A. et al. The spatiotemporal dynamics of microglia across the human lifespan. Dev. Cell 57, 2127–2139 (2022).
Ginhoux, F. & Prinz, M. Origin of microglia: current concepts and past controversies. Cold Spring Harb. Perspect. Biol. 7, a020537 (2015).
Mass, E. et al. Specification of tissue-resident macrophages during organogenesis. Science 353, aaf4238 (2016).
Ginhoux, F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010).
Askew, K. et al. Coupled proliferation and apoptosis maintain the rapid turnover of microglia in the adult brain. Cell Rep. 18, 391–405 (2017).
Lavin, Y. et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159, 1312–1326 (2014).
Gosselin, D. et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 159, 1327–1340 (2014). Together with Lavin et al. (2014), this work defines for the first time the repertoire of genomic regulatory elements in various subsets of tissue-resident macrophages and reveals how distal CREs interact with SDTFs in a tissue-dependent manner to specify cell identity of the different macrophage subsets.
Gautier, E. L. et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 13, 1118–1128 (2012).
Gosselin, D. et al. An environment-dependent transcriptional network specifies human microglia identity. Science 356, aal3222 (2017). This study characterizes for the first time the repertoire of genomic regulatory elements in human microglia, and identifies the key transcriptional regulators that enable their cell identity.
Butovsky, O. et al. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat. Neurosci. 17, 131–143 (2014). This work identifies for the first time how a soluble factor, TGFB, that is present in the brain promotes the microglial cell identity.
Galatro, T. F. et al. Transcriptomic analysis of purified human cortical microglia reveals age-associated changes. Nat. Neurosci. 20, 1162–1171 (2017).
Prinz, M., Jung, S. & Priller, J. Microglia biology: one century of evolving concepts. Cell 179, 292–311 (2019).
Butovsky, O. & Weiner, H. L. Microglial signatures and their role in health and disease. Nat. Rev. Neurosci. 19, 622–635 (2018).
Krasemann, S. et al. The TREM2–APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47, 566–581 (2017).
Keren-Shaul, H. et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290 (2017).
Hammond, T. R. et al. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity 50, 253–271 (2019).
Hasselmann, J. et al. Development of a chimeric model to study and manipulate human microglia in vivo. Neuron 103, 1016–1033 (2019).
Beckmann, N. et al. Brain region-specific enhancement of remyelination and prevention of demyelination by the CSF1R kinase inhibitor BLZ945. Acta Neuropathol. Commun. 6, 9 (2018).
Hagan, N. et al. CSF1R signaling is a regulator of pathogenesis in progressive MS. Cell Death Dis. 11, 904 (2020).
Hwang, D. et al. CSF-1 maintains pathogenic but not homeostatic myeloid cells in the central nervous system during autoimmune neuroinflammation. Proc. Natl Acad. Sci. USA 119, e2111804119 (2022).
Marzan, D. E. et al. Activated microglia drive demyelination via CSF1R signaling. Glia 69, 1583–1604 (2021).
Nissen, J. C., Thompson, K. K., West, B. L. & Tsirka, S. E. Csf1R inhibition attenuates experimental autoimmune encephalomyelitis and promotes recovery. Exp. Neurol. 307, 24–36 (2018).
Wies Mancini, V. S. B. et al. Colony-stimulating factor-1 receptor inhibition attenuates microgliosis and myelin loss but exacerbates neurodegeneration in the chronic cuprizone model. J. Neurochem. 160, 643–661 (2022).
Ransohoff, R. M. How neuroinflammation contributes to neurodegeneration. Science 353, 777–783 (2016).
Voet, S. et al. A20 critically controls microglia activation and inhibits inflammasome-dependent neuroinflammation. Nat. Commun. 9, 2036 (2018).
Liddelow, S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017). This comprehensive work provides insights into the molecular signals that trigger the acquisition of highly toxic functions by astrocytes in diseases.
Clark, I. C. et al. Barcoded viral tracing of single-cell interactions in central nervous system inflammation. Science 372, eabf1230 (2021).
Burda, J. E. et al. Divergent transcriptional regulation of astrocyte reactivity across disorders. Nature 606, 557–564 (2022).
Sofroniew, M. V. & Vinters, H. V. Astrocytes: biology and pathology. Acta Neuropathol. 119, 7–35 (2010).
Bretheau, F. et al. The alarmin interleukin-1α triggers secondary degeneration through reactive astrocytes and endothelium after spinal cord injury. Nat. Commun. 13, 5786 (2022).
Guttenplan, K. A. et al. Neurotoxic reactive astrocytes induce cell death via saturated lipids. Nature 599, 102–107 (2021).
Habbas, S. et al. Neuroinflammatory TNFα impairs memory via astrocyte signaling. Cell 163, 1730–1741 (2015).
Levesque, S. A. et al. Myeloid cell transmigration across the CNS vasculature triggers IL-1β-driven neuroinflammation during autoimmune encephalomyelitis in mice. J. Exp. Med. 213, 929–949 (2016).
Yamasaki, R. et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J. Exp. Med. 211, 1533–1549 (2014). This study provides compelling evidence demonstrating that monocytes and microglia interact differently with axons and myelin in demyelinating lesions.
Gao, H. et al. Opposing functions of microglial and macrophagic TNFR2 in the pathogenesis of experimental autoimmune encephalomyelitis. Cell Rep. 18, 198–212 (2017).
Pare, A. et al. IL-1β enables CNS access to CCR2hi monocytes and the generation of pathogenic cells through GM-CSF released by CNS endothelial cells. Proc. Natl Acad. Sci. USA 115, E1194–E1203 (2018).
Jordao, M. J. C. et al. Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science 363, eaat7554 (2019).
Haimon, Z. et al. Cognate microglia–T cell interactions shape the functional regulatory T cell pool in experimental autoimmune encephalomyelitis pathology. Nat. Immunol. 23, 1749–1762 (2022).
Wolf, Y. et al. Microglial MHC class II is dispensable for experimental autoimmune encephalomyelitis and cuprizone-induced demyelination. Eur. J. Immunol. 48, 1308–1318 (2018).
Kassiotis, G. & Kollias, G. Uncoupling the proinflammatory from the immunosuppressive properties of tumor necrosis factor (TNF) at the p55 TNF receptor level: implications for pathogenesis and therapy of autoimmune demyelination. J. Exp. Med. 193, 427–434 (2001).
Madsen, P. M. et al. Oligodendrocytes modulate the immune-inflammatory response in EAE via TNFR2 signaling. Brain Behav. Immun. 84, 132–146 (2020).
Madsen, P. M. et al. Oligodendroglial TNFR2 mediates membrane TNF-dependent repair in experimental autoimmune encephalomyelitis by promoting oligodendrocyte differentiation and remyelination. J. Neurosci. 36, 5128–5143 (2016).
Mailhot, B. et al. Neuronal interleukin-1 receptors mediate pain in chronic inflammatory diseases. J. Exp. Med. 217, e20191430 (2020).
Wheeler, M. A. et al. TNF-α/TNFR1 signaling is required for the development and function of primary nociceptors. Neuron 82, 587–602 (2014).
Binshtok, A. M. et al. Nociceptors are interleukin-1β sensors. J. Neurosci. 28, 14062–14073 (2008).
Ramaglia, V. et al. Complement-associated loss of CA2 inhibitory synapses in the demyelinated hippocampus impairs memory. Acta Neuropathol. 142, 643–667 (2021).
Werneburg, S. et al. Targeted complement inhibition at synapses prevents microglial synaptic engulfment and synapse loss in demyelinating disease. Immunity 52, 167–182 (2020). This investigation shows that excessive complement activity may contribute to aberrant synaptic remodelling and removal in demyelinating lesions.
Ramaglia, V. et al. C3-dependent mechanism of microglial priming relevant to multiple sclerosis. Proc. Natl Acad. Sci. USA 109, 965–970 (2012).
Szalai, A. J., Hu, X., Adams, J. E. & Barnum, S. R. Complement in experimental autoimmune encephalomyelitis revisited: C3 is required for development of maximal disease. Mol. Immunol. 44, 3132–3136 (2007).
Bourel, J. et al. Complement C3 mediates early hippocampal neurodegeneration and memory impairment in experimental multiple sclerosis. Neurobiol. Dis. 160, 105533 (2021).
Michailidou, I. et al. Complement C1q–C3-associated synaptic changes in multiple sclerosis hippocampus. Ann. Neurol. 77, 1007–1026 (2015).
Schafer, D. P. et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705 (2012). This study provides elegant evidence demonstrating that microglia are the key effector cells in the brain for the removal of synapses tagged by C1q complement factors.
Hong, S. et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352, 712–716 (2016).
Stevens, B. et al. The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178 (2007). This seminal work implicates for the first time the complement cascade in the development of neuronal circuitries in the brain.
Michailidou, I. et al. Complement C3 on microglial clusters in multiple sclerosis occur in chronic but not acute disease: implication for disease pathogenesis. Glia 65, 264–277 (2017).
Radi, R., Beckman, J. S., Bush, K. M. & Freeman, B. A. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch. Biochem. Biophys. 288, 481–487 (1991).
Li, J., Baud, O., Vartanian, T., Volpe, J. J. & Rosenberg, P. A. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc. Natl Acad. Sci. USA 102, 9936–9941 (2005).
Cross, A. H., Manning, P. T., Keeling, R. M., Schmidt, R. E. & Misko, T. P. Peroxynitrite formation within the central nervous system in active multiple sclerosis. J. Neuroimmunol. 88, 45–56 (1998).
Haider, L. et al. Oxidative damage in multiple sclerosis lesions. Brain 134, 1914–1924 (2011).
Nikic, I. et al. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat. Med. 17, 495–499 (2011).
Michaels, N. J. et al. Aging-exacerbated acute axon and myelin injury is associated with microglia-derived reactive oxygen species and is alleviated by the generic medication indapamide. J. Neurosci. 40, 8587–8600 (2020).
Mendiola, A. S. et al. Transcriptional profiling and therapeutic targeting of oxidative stress in neuroinflammation. Nat. Immunol. 21, 513–524 (2020).
Lambeth, J. D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4, 181–189 (2004).
Hu, C. F. et al. Microglial Nox2 plays a key role in the pathogenesis of experimental autoimmune encephalomyelitis. Front. Immunol. 12, 638381 (2021).
Ravelli, K. G. et al. Nox2-dependent neuroinflammation in an EAE model of multiple sclerosis. Transl. Neurosci. 10, 1–9 (2019).
Allan, E. R. et al. NADPH oxidase modifies patterns of MHC class II-restricted epitopic repertoires through redox control of antigen processing. J. Immunol. 192, 4989–5001 (2014).
Fischer, M. T. et al. NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain 135, 886–899 (2012).
Bagnato, F. et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain 134, 3602–3615 (2011).
Hametner, S. et al. Iron and neurodegeneration in the multiple sclerosis brain. Ann. Neurol. 74, 848–861 (2013).
Stephenson, E., Nathoo, N., Mahjoub, Y., Dunn, J. F. & Yong, V. W. Iron in multiple sclerosis: roles in neurodegeneration and repair. Nat. Rev. Neurol. 10, 459–468 (2014).
Connor, J. R. & Menzies, S. L. Cellular management of iron in the brain. J. Neurol. Sci. 134, 33–44 (1995).
Soares, M. P. & Hamza, I. Macrophages and iron metabolism. Immunity 44, 492–504 (2016).
Kenkhuis, B. et al. Iron loading is a prominent feature of activated microglia in Alzheimer’s disease patients. Acta Neuropathol. Commun. 9, 27 (2021).
Rodriguez-Callejas, J. D. et al. Loss of ferritin-positive microglia relates to increased iron, RNA oxidation, and dystrophic microglia in the brains of aged male marmosets. Am. J. Primatol. 81, e22956 (2019).
Lampron, A. et al. Inefficient clearance of myelin debris by microglia impairs remyelinating processes. J. Exp. Med. 212, 481–495 (2015).
Lloyd, A. F. et al. Central nervous system regeneration is driven by microglia necroptosis and repopulation. Nat. Neurosci. 22, 1046–1052 (2019).
Cantuti-Castelvetri, L. et al. Defective cholesterol clearance limits remyelination in the aged central nervous system. Science 359, 684–688 (2018). This study proposes a mechanism that explains how prolonged endocytic activity of microglia may incapacitate their ability to efficiently dispose of their internalized cargo, causing aberrent excessive inflammatory, tissue-damaging activity.
Bosch-Queralt, M. et al. Diet-dependent regulation of TGFβ impairs reparative innate immune responses after demyelination. Nat. Metab. 3, 211–227 (2021).
Miron, V. E. et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 16, 1211–1218 (2013). This work provides compelling evidence demonstrating how different states of microglia and macrophage activity can promote remyelination.
Dong, Y. et al. Oxidized phosphatidylcholines found in multiple sclerosis lesions mediate neurodegeneration and are neutralized by microglia. Nat. Neurosci. 24, 489–503 (2021). This work shows that oxidized phospholipids are highly toxic to myelin, and that their removal by microglia in a TREM2-dependent manner confers neuroprotection in demyelinating lesions.
Chen, M. S. et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 403, 434–439 (2000).
Schwab, M. E. & Caroni, P. Oligodendrocytes and CNS myelin are nonpermissive substrates for neurite growth and fibroblast spreading in vitro. J. Neurosci. 8, 2381–2393 (1988).
Kotter, M. R., Li, W. W., Zhao, C. & Franklin, R. J. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J. Neurosci. 26, 328–332 (2006).
Chuang, T. Y. et al. LRP1 expression in microglia is protective during CNS autoimmunity. Acta Neuropathol. Commun. 4, 68 (2016).
Butler, C. A. et al. Microglial phagocytosis of neurons in neurodegeneration, and its regulation. J. Neurochem. 158, 621–639 (2021).
Shen, K. et al. Multiple sclerosis risk gene Mertk is required for microglial activation and subsequent remyelination. Cell Rep. 34, 108835 (2021).
International Multiple Sclerosis Genetics Consortium et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 476, 214–219 (2011).
Weinger, J. G. et al. Loss of the receptor tyrosine kinase Axl leads to enhanced inflammation in the CNS and delayed removal of myelin debris during experimental autoimmune encephalomyelitis. J. Neuroinflammation 8, 49 (2011).
Binder, M. D. et al. Gas6 deficiency increases oligodendrocyte loss and microglial activation in response to cuprizone-induced demyelination. J. Neurosci. 28, 5195–5206 (2008).
Westman, J., Grinstein, S. & Marques, P. E. Phagocytosis of necrotic debris at sites of injury and inflammation. Front. Immunol. 10, 3030 (2019).
Safaiyan, S. et al. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat. Neurosci. 19, 995–998 (2016).
Storti, F. et al. Impaired ABCA1/ABCG1-mediated lipid efflux in the mouse retinal pigment epithelium (RPE) leads to retinal degeneration. eLife 8, e45100 (2019).
Dove, D. E., Linton, M. F. & Fazio, S. ApoE-mediated cholesterol efflux from macrophages: separation of autocrine and paracrine effects. Am. J. Physiol. Cell Physiol. 288, C586–C592 (2005).
Lin, C. Y., Duan, H. & Mazzone, T. Apolipoprotein E-dependent cholesterol efflux from macrophages: kinetic study and divergent mechanisms for endogenous versus exogenous apolipoprotein E. J. Lipid Res. 40, 1618–1627 (1999).
Hirsch-Reinshagen, V. et al. Deficiency of ABCA1 impairs apolipoprotein E metabolism in brain. J. Biol. Chem. 279, 41197–41207 (2004).
Meyers, E. A. & Kessler, J. A. TGF-β family signaling in neural and neuronal differentiation, development, and function. Cold Spring Harb. Perspect. Biol. 9, a022244 (2017).
Hayakawa, K. et al. Vascular endothelial growth factor regulates the migration of oligodendrocyte precursor cells. J. Neurosci. 31, 10666–10670 (2011).
Hill, R. A., Patel, K. D., Medved, J., Reiss, A. M. & Nishiyama, A. NG2 cells in white matter but not gray matter proliferate in response to PDGF. J. Neurosci. 33, 14558–14566 (2013).
Wlodarczyk, A. et al. A novel microglial subset plays a key role in myelinogenesis in developing brain. EMBO J. 36, 3292–3308 (2017).
McKinnon, R. D., Piras, G., Ida, J. A. Jr. & Dubois-Dalcq, M. A role for TGF-β in oligodendrocyte differentiation. J. Cell Biol. 121, 1397–1407 (1993).
Locatelli, G. et al. IGF1R expression by adult oligodendrocytes is not required in the steady-state but supports neuroinflammation. Glia 71, 616–632 (2022).
DiToro, D. et al. Insulin-like growth factors are key regulators of T helper 17 regulatory T cell balance in autoimmunity. Immunity 52, 650–667 (2020).
Berghoff, S. A. et al. Microglia facilitate repair of demyelinated lesions via post-squalene sterol synthesis. Nat. Neurosci. 24, 47–60 (2021). This work provides compelling evidence demonstrating how desmosterol and cholesterol processing in microglia may support myelin repair in demyelinating lesions.
Berghoff, S. A., Spieth, L. & Saher, G. Local cholesterol metabolism orchestrates remyelination. Trends Neurosci. 45, 272–283 (2022).
Sherafat, A., Pfeiffer, F., Reiss, A. M., Wood, W. M. & Nishiyama, A. Microglial neuropilin-1 promotes oligodendrocyte expansion during development and remyelination by trans-activating platelet-derived growth factor receptor. Nat. Commun. 12, 2265 (2021).
Giera, S. et al. Microglial transglutaminase-2 drives myelination and myelin repair via GPR56/ADGRG1 in oligodendrocyte precursor cells. eLife 7, e33385 (2018).
Baror, R. et al. Transforming growth factor-β renders ageing microglia inhibitory to oligodendrocyte generation by CNS progenitors. Glia 67, 1374–1384 (2019).
Huang, W., Bai, X., Meyer, E. & Scheller, A. Acute brain injuries trigger microglia as an additional source of the proteoglycan NG2. Acta Neuropathol. Commun. 8, 146 (2020).
Lau, L. W. et al. Chondroitin sulfate proteoglycans in demyelinated lesions impair remyelination. Ann. Neurol. 72, 419–432 (2012).
Skuljec, J. et al. Matrix metalloproteinases and their tissue inhibitors in cuprizone-induced demyelination and remyelination of brain white and gray matter. J. Neuropathol. Exp. Neurol. 70, 758–769 (2011).
Rempe, R. G., Hartz, A. M. S. & Bauer, B. Matrix metalloproteinases in the brain and blood–brain barrier: versatile breakers and makers. J. Cereb. Blood Flow. Metab. 36, 1481–1507 (2016).
Goncalves DaSilva, A. & Yong, V. W. Matrix metalloproteinase-12 deficiency worsens relapsing–remitting experimental autoimmune encephalomyelitis in association with cytokine and chemokine dysregulation. Am. J. Pathol. 174, 898–909 (2009).
Madala, S. K. et al. Matrix metalloproteinase 12-deficiency augments extracellular matrix degrading metalloproteinases and attenuates IL-13-dependent fibrosis. J. Immunol. 184, 3955–3963 (2010).
Kaufmann, M. et al. Identification of early neurodegenerative pathways in progressive multiple sclerosis. Nat. Neurosci. 25, 944–955 (2022).
Buttgereit, A. et al. Sall1 is a transcriptional regulator defining microglia identity and function. Nat. Immunol. 17, 1397–1406 (2016).
International Multiple Sclerosis Genetics, C. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science 365, aav7188 (2019).
Bryois, J. et al. Cell-type-specific cis-eQTLs in eight human brain cell types identify novel risk genes for psychiatric and neurological disorders. Nat. Neurosci. 25, 1104–1112 (2022). This significant work comprehensively catalogues for the first time on a genome-wide level the genetic risk factors for MS that may disrupt microglial functions by interfering with activity at their distal CREs.
Davalos, D. et al. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat. Commun. 3, 1227 (2012).
Petersen, M. A., Ryu, J. K. & Akassoglou, K. Fibrinogen in neurological diseases: mechanisms, imaging and therapeutics. Nat. Rev. Neurosci. 19, 283–301 (2018).
Adams, R. A. et al. The fibrin-derived γ377–395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J. Exp. Med. 204, 571–582 (2007).
Ryu, J. K. et al. Fibrin-targeting immunotherapy protects against neuroinflammation and neurodegeneration. Nat. Immunol. 19, 1212–1223 (2018). Together with Adams et al. (2007), this work demonstrates how vascular leakage of fibrin may trigger neuroinflammatory responses in the CNS.
Kirk, J., Plumb, J., Mirakhur, M. & McQuaid, S. Tight junctional abnormality in multiple sclerosis white matter affects all calibres of vessel and is associated with blood–brain barrier leakage and active demyelination. J. Pathol. 201, 319–327 (2003).
Baecher-Allan, C., Kaskow, B. J. & Weiner, H. L. Multiple sclerosis: mechanisms and immunotherapy. Neuron 97, 742–768 (2018).
Koizumi, S. et al. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature 446, 1091–1095 (2007).
Davalos, D. et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8, 752–758 (2005).
Jaitin, D. A. et al. Lipid-associated macrophages control metabolic homeostasis in a Trem2-dependent manner. Cell 178, 686–698 (2019).
Wang, Y. et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell 160, 1061–1071 (2015).
Cantoni, C. et al. TREM2 regulates microglial cell activation in response to demyelination in vivo. Acta Neuropathol. 129, 429–447 (2015).
Poliani, P. L. et al. TREM2 sustains microglial expansion during aging and response to demyelination. J. Clin. Invest. 125, 2161–2170 (2015). Together with Cantoni et al. (2015), this work demonstrates for the first time the critical role of TREM2 signalling in coordinating microglial inflammatory responses in demyelinating lesions.
Cignarella, F. et al. TREM2 activation on microglia promotes myelin debris clearance and remyelination in a model of multiple sclerosis. Acta Neuropathol. 140, 513–534 (2020).
Nugent, A. A. et al. TREM2 regulates microglial cholesterol metabolism upon chronic phagocytic challenge. Neuron 105, 837–854 (2020).
Gouna, G. et al. TREM2-dependent lipid droplet biogenesis in phagocytes is required for remyelination. J. Exp. Med. 218, e20210227 (2021).
Dong, Y. & Yong, V. W. Oxidized phospholipids as novel mediators of neurodegeneration. Trends Neurosci. 45, 419–429 (2022).
Atagi, Y. et al. Apolipoprotein E is a ligand for triggering receptor expressed on myeloid cells 2 (TREM2). J. Biol. Chem. 290, 26043–26050 (2015).
Cochain, C. et al. Single-cell RNA-seq reveals the transcriptional landscape and heterogeneity of aortic macrophages in murine atherosclerosis. Circ. Res. 122, 1661–1674 (2018).
Piccio, L. et al. Blockade of TREM-2 exacerbates experimental autoimmune encephalomyelitis. Eur. J. Immunol. 37, 1290–1301 (2007).
Hannun, Y. A. & Obeid, L. M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 19, 175–191 (2018).
Alaamery, M. et al. Role of sphingolipid metabolism in neurodegeneration. J. Neurochem. 158, 25–35 (2021).
Kappos, L. et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N. Engl. J. Med. 362, 387–401 (2010).
Calabresi, P. A. et al. Safety and efficacy of fingolimod in patients with relapsing–remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 13, 545–556 (2014).
Groves, A., Kihara, Y. & Chun, J. Fingolimod: direct CNS effects of sphingosine 1-phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy. J. Neurol. Sci. 328, 9–18 (2013).
Colombo, E. & Farina, C. Lessons from S1P receptor targeting in multiple sclerosis. Pharmacol. Ther. 230, 107971 (2022).
Kim, S. et al. Functional antagonism of sphingosine-1-phosphate receptor 1 prevents cuprizone-induced demyelination. Glia 66, 654–669 (2018).
Kim, H. J. et al. Neurobiological effects of sphingosine 1-phosphate receptor modulation in the cuprizone model. Faseb J. 25, 1509–1518 (2011).
Jackson, S. J., Giovannoni, G. & Baker, D. Fingolimod modulates microglial activation to augment markers of remyelination. J. Neuroinflammation 8, 76 (2011).
Bogie, J. F. J. et al. Stearoyl-CoA desaturase-1 impairs the reparative properties of macrophages and microglia in the brain. J. Exp. Med. 217, e20191660 (2020).
Duewell, P. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010).
Chu, T. T. et al. Tonic prime-boost of STING signalling mediates Niemann–Pick disease type C. Nature 596, 570–575 (2021).
Kollias, G., Douni, E., Kassiotis, G. & Kontoyiannis, D. The function of tumour necrosis factor and receptors in models of multi-organ inflammation, rheumatoid arthritis, multiple sclerosis and inflammatory bowel disease. Ann. Rheum. Dis. 58, I32–I39 (1999).
Witmer-Pack, M. D. et al. Identification of macrophages and dendritic cells in the osteopetrotic (op/op) mouse. J. Cell Sci. 104, 1021–1029 (1993).
De, I. et al. CSF1 overexpression has pleiotropic effects on microglia in vivo. Glia 62, 1955–1967 (2014).
Wegiel, J. et al. Reduced number and altered morphology of microglial cells in colony stimulating factor-1-deficient osteopetrotic op/op mice. Brain Res. 804, 135–139 (1998).
Gomez-Nicola, D., Fransen, N. L., Suzzi, S. & Perry, V. H. Regulation of microglial proliferation during chronic neurodegeneration. J. Neurosci. 33, 2481–2493 (2013).
Tanabe, S., Saitoh, S., Miyajima, H., Itokazu, T. & Yamashita, T. Microglia suppress the secondary progression of autoimmune encephalomyelitis. Glia 67, 1694–1704 (2019).
Wlodarczyk, A. et al. CSF1R stimulation promotes increased neuroprotection by CD11c+ microglia in EAE. Front. Cell Neurosci. 12, 523 (2018).
Laflamme, N. et al. mCSF-induced microglial activation prevents myelin loss and promotes its repair in a mouse model of multiple sclerosis. Front. Cell Neurosci. 12, 178 (2018).
Kocur, M. et al. IFNβ secreted by microglia mediates clearance of myelin debris in CNS autoimmunity. Acta Neuropathol. Commun. 3, 20 (2015).
Traugott, U. & Lebon, P. Multiple sclerosis: involvement of interferons in lesion pathogenesis. Ann. Neurol. 24, 243–251 (1988).
McNab, F., Mayer-Barber, K., Sher, A., Wack, A. & O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 15, 87–103 (2015).
Ivashkiv, L. B. & Donlin, L. T. Regulation of type I interferon responses. Nat. Rev. Immunol. 14, 36–49 (2014).
Prinz, M. et al. Distinct and nonredundant in vivo functions of IFNAR on myeloid cells limit autoimmunity in the central nervous system. Immunity 28, 675–686 (2008).
Goldmann, T. et al. USP18 lack in microglia causes destructive interferonopathy of the mouse brain. EMBO J. 34, 1612–1629 (2015).
Schmidt, H. et al. Type I interferon receptor signalling is induced during demyelination while its function for myelin damage and repair is redundant. Exp. Neurol. 216, 306–311 (2009).
Trebst, C. et al. Lack of interferon-β leads to accelerated remyelination in a toxic model of central nervous system demyelination. Acta Neuropathol. 114, 587–596 (2007).
Csumita, M. et al. Specific enhancer selection by IRF3, IRF5 and IRF9 is determined by ISRE half-sites, 5′ and 3′ flanking bases, collaborating transcription factors and the chromatin environment in a combinatorial fashion. Nucleic Acids Res. 48, 589–604 (2020).
Rothlin, C. V., Ghosh, S., Zuniga, E. I., Oldstone, M. B. & Lemke, G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell 131, 1124–1136 (2007).
Lemke, G. Biology of the TAM receptors. Cold Spring Harb. Perspect. Biol. 5, a009076 (2013).
Yan, Z. et al. Deficiency of Socs3 leads to brain-targeted EAE via enhanced neutrophil activation and ROS production. JCI Insight 5, e126520 (2019).
Chinetti, G. et al. PPAR-α and PPAR-γ activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat. Med. 7, 53–58 (2001).
Bogie, J. F. et al. Myelin alters the inflammatory phenotype of macrophages by activating PPARs. Acta Neuropathol. Commun. 1, 43 (2013).
Straus, D. S. & Glass, C. K. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 28, 551–558 (2007).
Doroshenko, E. R. et al. Peroxisome proliferator-activated receptor-δ deficiency in microglia results in exacerbated axonal injury and tissue loss in experimental autoimmune encephalomyelitis. Front. Immunol. 12, 570425 (2021).
Boven, L. A. et al. Myelin-laden macrophages are anti-inflammatory, consistent with foam cells in multiple sclerosis. Brain 129, 517–526 (2006).
Jovanovic, M. et al. Immunogenetics. Dynamic profiling of the protein life cycle in response to pathogens. Science 347, 1259038 (2015).
Lee, M. J. et al. IKKβ-mediated inflammatory myeloid cell activation exacerbates experimental autoimmune encephalomyelitis by potentiating TH1/TH17 cell activation and compromising blood brain barrier. Mol. Neurodegener. 11, 54 (2016).
Goldmann, T. et al. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat. Neurosci. 16, 1618–1626 (2013).
Jie, Z. et al. Microglia promote autoimmune inflammation via the noncanonical NF-κB pathway. Sci. Adv. 7, eabh0609 (2021).
Ennerfelt, H. et al. SYK coordinates neuroprotective microglial responses in neurodegenerative disease. Cell 185, 4135–4152 (2022).
Montalban, X. et al. Placebo-controlled trial of an oral BTK inhibitor in multiple sclerosis. N. Engl. J. Med. 380, 2406–2417 (2019).
Reich, D. S. et al. Safety and efficacy of tolebrutinib, an oral brain-penetrant BTK inhibitor, in relapsing multiple sclerosis: a phase 2b, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 20, 729–738 (2021).
Baba, Y. et al. BLNK mediates Syk-dependent Btk activation. Proc. Natl Acad. Sci. USA 98, 2582–2586 (2001).
Hendriks, R. W., Yuvaraj, S. & Kil, L. P. Targeting Bruton’s tyrosine kinase in B cell malignancies. Nat. Rev. Cancer 14, 219–232 (2014).
Pellerin, K. et al. MOG autoantibodies trigger a tightly-controlled FcR and BTK-driven microglia proliferative response. Brain 144, 2361–2374 (2021).
Middendorp, S. et al. Tumor suppressor function of Bruton tyrosine kinase is independent of its catalytic activity. Blood 105, 259–265 (2005).
Nam, H. Y. et al. Ibrutinib suppresses LPS-induced neuroinflammatory responses in BV2 microglial cells and wild-type mice. J. Neuroinflammation 15, 271 (2018).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Ghisletti, S. et al. Identification and characterization of enhancers controlling the inflammatory gene expression program in macrophages. Immunity 32, 317–328 (2010). Together with Heinz et al. (2010), this work characterizes for the first time the repertoire of distal CREs in macrophages, and provides evidence for a general, hierarchical model for the selection and function of cell-type-specific enhancers.
Ostuni, R. et al. Latent enhancers activated by stimulation in differentiated cells. Cell 152, 157–171 (2013).
Karr, J. P., Ferrie, J. J., Tjian, R. & Darzacq, X. The transcription factor activity gradient (TAG) model: contemplating a contact-independent mechanism for enhancer–promoter communication. Genes Dev. 36, 7–16 (2022).
Ren, B. & Yue, F. Transcriptional enhancers: bridging the genome and phenome. Cold Spring Harb. Symp. Quant. Biol. 80, 17–26 (2015).
Xavier, A. M. et al. Systematic delineation of signaling and epigenomic mechanisms underlying microglia inflammatory activity in acute and chronic brain pathologies. Preprint at bioRxiv https://www.biorxiv.org/content/10.1101/2022.08.04.502805v1 (2022).
Perera, R. M., Di Malta, C. & Ballabio, A. MiT/TFE family of transcription factors, lysosomes, and cancer. Annu. Rev. Cancer Biol. 3, 203–222 (2019).
Haney, M. S. et al. Identification of phagocytosis regulators using magnetic genome-wide CRISPR screens. Nat. Genet. 50, 1716–1727 (2018).
Daniel, B. et al. The transcription factor EGR2 is the molecular linchpin connecting STAT6 activation to the late, stable epigenomic program of alternative macrophage polarization. Genes. Dev. 34, 1474–1492 (2020).
Belhocine, S. et al. Context-dependent transcriptional regulation of microglial proliferation. Glia 70, 572–589 (2022).
Glass, C. K. & Natoli, G. Molecular control of activation and priming in macrophages. Nat. Immunol. 17, 26–33 (2016).
Mishra, M. K. et al. Harnessing the benefits of neuroinflammation: generation of macrophages/microglia with prominent remyelinating properties. J. Neurosci. 41, 3366–3385 (2021).
Heinz, S., Romanoski, C. E., Benner, C. & Glass, C. K. The selection and function of cell type-specific enhancers. Nat. Rev. Mol. Cell Biol. 16, 144–154 (2015).
Heinz, S. et al. Effect of natural genetic variation on enhancer selection and function. Nature 503, 487–492 (2013).
Saeed, S. et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345, 1251086 (2014).
Novakovic, B. et al. β-Glucan reverses the epigenetic state of LPS-induced immunological tolerance. Cell 167, 1354–1368 (2016).
Zhang, X. et al. Epigenetic regulation of innate immune memory in microglia. J. Neuroinflammation 19, 111 (2022).
Wendeln, A. C. et al. Innate immune memory in the brain shapes neurological disease hallmarks. Nature 556, 332–338 (2018).
Kaikkonen, M. U. et al. Remodeling of the enhancer landscape during macrophage activation is coupled to enhancer transcription. Mol. Cell 51, 310–325 (2013).
Cheng, Q. J. et al. NF-κB dynamics determine the stimulus specificity of epigenomic reprogramming in macrophages. Science 372, 1349–1353 (2021).
Mehta, V. et al. Iron is a sensitive biomarker for inflammation in multiple sclerosis lesions. PLoS ONE 8, e57573 (2013).
Schuh, C. et al. Oxidative tissue injury in multiple sclerosis is only partly reflected in experimental disease models. Acta Neuropathol. 128, 247–266 (2014).
Pandur, E. et al. Relationship of iron metabolism and short-term cuprizone treatment of C57BL/6 mice. Int. J. Mol. Sci. 20, 2257 (2019).
Bjornevik, K. et al. Longitudinal analysis reveals high prevalence of Epstein–Barr virus associated with multiple sclerosis. Science 375, 296–301 (2022). This study provides the most robust evidence to date implicating EBV infection as a causative factor in MS aetiology.
Wirtz, T. et al. Mouse model for acute Epstein–Barr virus infection. Proc. Natl Acad. Sci. USA 113, 13821–13826 (2016).
Li, R., Patterson, K. R. & Bar-Or, A. Reassessing B cell contributions in multiple sclerosis. Nat. Immunol. 19, 696–707 (2018).
Jain, R. W. & Yong, V. W. B cells in central nervous system disease: diversity, locations and pathophysiology. Nat. Rev. Immunol. 22, 513–524 (2022).
Weber, M. S. et al. B-cell activation influences T-cell polarization and outcome of anti-CD20 B-cell depletion in central nervous system autoimmunity. Ann. Neurol. 68, 369–383 (2010).
Franklin, R. J. M. & Simons, M. CNS remyelination and inflammation: from basic mechanisms to therapeutic opportunities. Neuron 110, 3549–3565 (2022).
Green, A. J. et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. Lancet 390, 2481–2489 (2017).
Brown, J. W. L. et al. Safety and efficacy of bexarotene in patients with relapsing-remitting multiple sclerosis (CCMR One): a randomised, double-blind, placebo-controlled, parallel-group, phase 2a study. Lancet Neurol. 20, 709–720 (2021).
Brown, J. W. L. et al. Remyelination varies between and within lesions in multiple sclerosis following bexarotene. Ann. Clin. Transl. Neurol. 9, 1626–1642 (2022).
Roszer, T., Menendez-Gutierrez, M. P., Cedenilla, M. & Ricote, M. Retinoid X receptors in macrophage biology. Trends Endocrinol. Metab. 24, 460–468 (2013).
Mundt, S., Greter, M. & Becher, B. The CNS mononuclear phagocyte system in health and disease. Neuron 110, 3497–3512 (2022).
Kierdorf, K., Masuda, T., Jordao, M. J. C. & Prinz, M. Macrophages at CNS interfaces: ontogeny and function in health and disease. Nat. Rev. Neurosci. 20, 547–562 (2019).
Mildenberger, W., Stifter, S. A. & Greter, M. Diversity and function of brain-associated macrophages. Curr. Opin. Immunol. 76, 102181 (2022).
Lee, J. et al. QUAKING regulates microexon alternative splicing of the Rho GTPase pathway and controls microglia homeostasis. Cell Rep. 33, 108560 (2020).
Ren, J. et al. Qki is an essential regulator of microglial phagocytosis in demyelination. J. Exp. Med. 218, e201903 (2021).
Becher, B., Spath, S. & Goverman, J. Cytokine networks in neuroinflammation. Nat. Rev. Immunol. 17, 49–59 (2017).
Vela, J. M., Molina-Holgado, E., Arevalo-Martin, A., Almazan, G. & Guaza, C. Interleukin-1 regulates proliferation and differentiation of oligodendrocyte progenitor cells. Mol. Cell Neurosci. 20, 489–502 (2002).
Grajchen, E., Hendriks, J. J. A. & Bogie, J. F. J. The physiology of foamy phagocytes in multiple sclerosis. Acta Neuropathol. Commun. 6, 124 (2018).
Borggrewe, M. et al. VISTA regulates microglia homeostasis and myelin phagocytosis, and is associated with MS lesion pathology. Acta Neuropathol. Commun. 9, 91 (2021).
Ransohoff, R. M. Animal models of multiple sclerosis: the good, the bad and the bottom line. Nat. Neurosci. 15, 1074–1077 (2012).
Constantinescu, C. S., Farooqi, N., O’Brien, K. & Gran, B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br. J. Pharmacol. 164, 1079–1106 (2011).
Hoftberger, R. et al. The pathology of central nervous system inflammatory demyelinating disease accompanying myelin oligodendrocyte glycoprotein autoantibody. Acta Neuropathol. 139, 875–892 (2020).
T Hart, B. A., Gran, B. & Weissert, R. EAE: imperfect but useful models of multiple sclerosis. Trends Mol. Med. 17, 119–125 (2011).
Wagner, C. A., Roque, P. J., Mileur, T. R., Liggitt, D. & Goverman, J. M. Myelin-specific CD8+ T cells exacerbate brain inflammation in CNS autoimmunity. J. Clin. Invest. 130, 203–213 (2020).
Hauser, S. L. et al. Immunohistochemical analysis of the cellular infiltrate in multiple sclerosis lesions. Ann. Neurol. 19, 578–587 (1986).
Frischer, J. M. et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 132, 1175–1189 (2009).
Caravagna, C. et al. Diversity of innate immune cell subsets across spatial and temporal scales in an EAE mouse model. Sci. Rep. 8, 5146 (2018).
Lindsey, J. W. et al. Repeated treatment with chimeric anti-CD4 antibody in multiple sclerosis. Ann. Neurol. 36, 183–189 (1994).
van Oosten, B. W. et al. Treatment of multiple sclerosis with the monoclonal anti-CD4 antibody cM-T412: results of a randomized, double-blind, placebo-controlled, MR-monitored phase II trial. Neurology 49, 351–357 (1997).
Cobbold, S. P., Jayasuriya, A., Nash, A., Prospero, T. D. & Waldmann, H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature 312, 548–551 (1984).
Dang, A. K., Jain, R. W., Craig, H. C. & Kerfoot, S. M. B cell recognition of myelin oligodendrocyte glycoprotein autoantigen depends on immunization with protein rather than short peptide, while B cell invasion of the CNS in autoimmunity does not. J. Neuroimmunol. 278, 73–84 (2015).
Jain, R. W. et al. Autoreactive, low-affinity T cells preferentially drive differentiation of short-lived memory B cells at the expense of germinal center maintenance. Cell Rep. 25, 3342–3355 (2018).
Hauser, S. L. et al. Ocrelizumab versus interferon β-1a in relapsing multiple sclerosis. N. Engl. J. Med. 376, 221–234 (2017).
Hauser, S. L. et al. Ofatumumab versus teriflunomide in multiple sclerosis. N. Engl. J. Med. 383, 546–557 (2020).
Zirngibl, M., Assinck, P., Sizov, A., Caprariello, A. V. & Plemel, J. R. Oligodendrocyte death and myelin loss in the cuprizone model: an updated overview of the intrinsic and extrinsic causes of cuprizone demyelination. Mol. Neurodegener. 17, 34 (2022).
Denic, A. et al. The relevance of animal models in multiple sclerosis research. Pathophysiology 18, 21–29 (2011).
Acknowledgements
The authors thank A. Boisvert for assistance with manuscript editing, and M. Toghi for assistance with preparation of figures. F.D.-G. is supported by a Doctoral award from the Canadian Institutes of Health Research (CIHR). This work was supported by grants awarded to D.G. from the Scottish Rite Charitable Foundation of Canada, Multiple Sclerosis Society of Canada, Alzheimer’s Society of Canada and CIHR.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neuroscience thanks L. Piccio and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Antigen presentation
-
A process whereby antigens bound to a cell’s major histocompatibility complex (MHC) class I or II surface receptors are presented, respectively, to CD8 or CD4 lymphocytes to induce their activation.
- Chemokines
-
Soluble proteins that mediate recruitment of cells through chemotaxis.
- Cis-regulatory elements
-
(CREs). Genomic regulatory elements that encompass binding sites for transcription factors implicated in the regulation of associated genes.
- Complement pathway
-
A coordinated protein cascade involved in immune defence; also increasingly involved in mediating essential functions in the development and maintenance of neuronal circuitries.
- Cytokine
-
A soluble protein that mediates cell-to-cell communication.
- Damage-associated molecular patterns
-
(DAMPs). Endogenous molecules typically released by injured cells that are endowed with the ability to trigger potent inflammatory responses.
- Demyelinating lesions
-
Lesions of the nervous system characterized by damage and/or loss of myelin on axons.
- Endolysosomal system
-
A system of cell biology that mediates trafficking, recycling and/or disposal through proteolysis of molecular cargo contained within membranous organelles.
- Gene promoters
-
Genomic regulatory elements that encompass transcriptional start sites of protein-coding genes along with binding sites for transcription factors involved in the recruitment and positioning of the RNA polymerase II transcriptional complex.
- Genetic susceptibility variants
-
Genetic alterations that increase risk for the development of a disease or disorder.
- Inflammasome
-
A protein complex that cleaves the pro-form of interleukin-1β (IL-1B) and other IL-1 family members to produce their mature, signalling forms.
- Lipofuscin
-
Granules, largely composed of lipid remains, that may form and persist over time in lysosomes under certain circumstances.
- Multiplexed protein imaging
-
Techniques that enable the detection and quantification of multiple target proteins in parallel, or serially, in situ.
- Opsonization
-
A process whereby proteins bind and coat targeted structures to enable and/or facilitate their disposal.
- Phagocytosis
-
A form of endocytosis involved in the internalization of particles larger than 0.5 μm in diameter.
- Reactive oxygen species
-
(ROS). Highly reactive chemicals produced through the partial reduction of oxygen.
- Scavenger receptors
-
A diverse group of cell surface receptors that mediate endocytosis of a wide variety of particles, including lipids, carbohydrates and apoptotic cells.
- Single-cell RNA sequencing
-
A gene profiling technique that allows the comprehensive assessment of relative gene expression at the single-cell level.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Distéfano-Gagné, F., Bitarafan, S., Lacroix, S. et al. Roles and regulation of microglia activity in multiple sclerosis: insights from animal models. Nat Rev Neurosci 24, 397–415 (2023). https://doi.org/10.1038/s41583-023-00709-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-023-00709-6