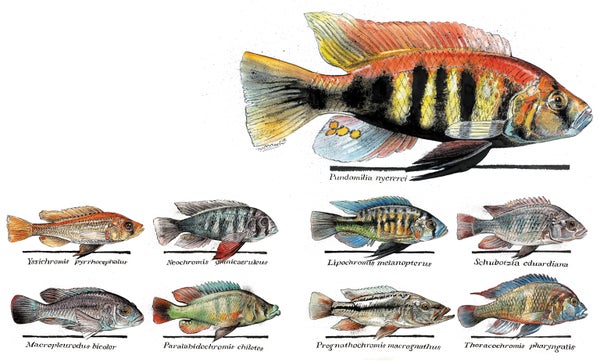
Africa's Lake Victoria is home to one of evolution's greatest experiments. In its waters, what began as a single lineage belonging to the cichlid family of fishes has since given rise to a dazzling array of forms. Like Charles Darwin's famous finches, which evolved a wide range of beak shapes and sizes to exploit the different foods available in the Galápagos Islands, these cichlids represent a textbook example of what biologists term an adaptive radiation—the phenomenon whereby one lineage spawns numerous species that evolve specializations to an array of ecological roles. But the Lake Victoria cichlids far surpass Darwin's finches in the astonishing speed with which they diversified: the more than 500 species that live there and only there today all evolved within the past 15,000 to 10,000 years—an eyeblink in geologic terms—compared with the 14 finch species that evolved over several million years.
Lake Victoria is not the only locale cichlids call home. Other tropical freshwater lakes and rivers in Africa, as well as the Americas and the tip of the Indian subcontinent, harbor their own cichlids. All told, the family is estimated to comprise more than 2,500 species. Some, such as the tilapias, are farmed for food and are among the most important aquaculture species in the world. Most, like the oscars and angelfish, are popular with aquarium enthusiasts because they are beautiful and have many interesting courtship and parenting behaviors. Many species have yet to be formally described. The cichlids share their lakes with other families of fishes, but only cichlids have managed to speciate so extensively and so fast. Indeed, no other group of vertebrate animals can rival the cichlids in terms of sheer number of species and variety of body shape, coloration and behavior. At the same time, however, evolution has often repeated itself in these fishes: a number of the same adaptations have evolved in parallel in the separate cichlid lineages—a curious trend.
I and other scientists have long marveled at the manifold forms of cichlids and wondered what factors allowed this group to differentiate in such spectacular fashion. Recent advances in genome-sequencing technology have allowed us to comb their DNA for clues to this evolutionary exuberance. We have not solved the full puzzle of cichlid diversity yet—far from it. But we have found some special characteristics of cichlid genomes that might have primed them to diversify with such speed yet also to evolve certain traits again and again. As we explore the genetic underpinnings of the extraordinary success of this group of fishes, we are glimpsing the very cogs and wheels of evolution—insights that will help researchers decode the origins of all manner of species.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Different but the same
To get a clearer picture of just how amazingly diverse cichlids are, consider the forms in Lake Victoria and the two other lakes in which the major East African cichlid radiations occurred: Lake Malawi, which harbors perhaps 800 to 1,000 species, and Lake Tanganyika, with its roughly 250 species from older cichlid lineages, one of which colonized the other two younger lakes and spawned the radiations there. These cichlids display every rainbow hue and range from about an inch to three feet in length. And they have evolved adaptations to eating every conceivable food source in their environment. Algae scrapers have flat teeth like human incisors that allow them to nibble the nutritious growths on rock surfaces; insect eaters have long, pointy teeth that help them to get into rock crevices; ambush predators possess huge extendable jaws with which they can suck in their unsuspecting prey in a matter of milliseconds. Those are just some of the broader categories of specialization. Among the algae scrapers, for instance, some species are adapted to foraging in the wave-break zone, others to harvesting food from one particular pile of rocks and no other, and still others to feeding on certain angles from the rocks or only on specific types of algae.
And yet for all the variety seen in the East African cichlids, some highly specialized traits have evolved repeatedly—to a surprising degree. For instance, several species of cichlids in all three lakes feed almost exclusively on the scales of other fishes. They have all developed the same distinctive rakelike teeth that allow them to hold on to the scales of their victims. But their teeth are not the only adaptation to this diet. Their jaws have evolved to be asymmetrical, opening either to the left or to the right, but not both, so as to better grab the scales from a given side of their targets. “Left-headed” cichlids rasp the scales from the right side of their victim; “right-headed” ones scrape from the left. (Natural selection has kept these asymmetrical forms in balance: about half of the scale eaters that my colleagues and I collected around Lake Tanganyika were right-headed, and the other half were left-headed.) The scale eaters have a very particular set of adaptations to their way of life, but somehow these same traits have arisen at least three separate times in these East African lakes.

Credit: Jack Unruh
Another distinctive adaptation that has emerged independently multiple times is enlarged lips in species that target prey found in rock crevices. My colleagues and I have shown that these “Angelina Jolie” lips act as seals and bumpers that help the fishes suck prey out from their hiding spots. (Cichlids lacking such lips cannot reach prey efficiently in narrow crevices.) Remarkably, species in all three African radiations, as well as two New World radiations, have developed this trait in parallel.
Similarly, distinctive coloration patterns have evolved independently in several separate cichlid lineages. Whereas the vast majority of cichlids sport dark, vertical stripes that presumably camouflage them from predators, a handful of species from each of the three East African lakes also evolved horizontal stripes. This strikingly different patterning occurs mostly in open-water species that tend to be fast swimmers and predators, possibly because the horizontal stripes help to obscure their body shapes from watchful prey.
Engines of innovation
The seemingly contradictory themes of extreme diversification and repeated parallel evolution of ultraspecialized adaptations in cichlid evolution raise several key questions. One begins with the highly specialized eaters and the observation that, ordinarily, adapting to a narrow diet means that if something goes wrong with the needed food source, the specialist is in trouble. So how have the cichlids managed to avoid falling into that trap? One answer seems to be a strange anatomical feature that cichlids alone possess among freshwater fishes. All cichlids have a normal pair of mouth jaws and a second pair of jaws located in the throat, like the monster in the movie Alien. Any food they eat gets grabbed and processed first by the mouth jaws and second by the throat jaws. As a result, cichlids can have mouth jaws adapted to one kind of food, and their throat jaws can break down other stuff. In this novel way, many cichlids have become jacks-of-all-trades, masters-of-one. In other words, they can evolve a specialization but remain generalists at the same time in case their preferred food runs out or a better option becomes available.
The throat jaws explain how cichlids have been able to mitigate the risk of specializing, but what was the source of all the evolutionary novelty in these fishes? What were the factors that spurred the genes that encode their traits to change so rapidly, and how did the same adaptations keep turning up in separate lineages? Recently, thanks to the advent of fast genome-sequencing methods for health research, my colleagues and I—a consortium of more than 70 researchers from laboratories around the world, led by the Broad Institute in Cambridge, Mass.—have begun making inroads into answering these questions and solving the riddle of the cichlid family's stunning success. In 2014, for the first time, we decoded the sequence of DNA code letters in cichlid genomes. We obtained complete sequences for five African species and partial sequences for 60 individuals representing six very closely related species from Lake Victoria alone. By comparing these genomes with one another and with those of the cichlids' relatives, the sticklebacks—a far less diverse family of fishes—we have been able to identify features of cichlid genomes that help to explain the group's diversity.
Among the first things the Cichlid Genome Consortium looked for in these genomes were mutations that have produced changes in the amino acids that make up proteins; proteins do much of the work in cells, and many genes specify the sequence of amino acids that get strung together to generate a given protein. An overabundance of proteins containing altered amino acids would suggest that the genes harboring the underlying mutations were under strong selection pressure to evolve quickly; that is, conditions were such that fishes that acquired certain amino acid changes thereby gained a strong survival or reproductive advantage. We found that even the tilapia species we sequenced, which is an evolutionarily unremarkable cichlid compared with its brethren, had more such mutations than the sticklebacks. And the cichlids from the hyperdiverse groups in Lake Malawi and Lake Victoria had mutation rates several times higher than the tilapia's. Many of the affected genes are known to be involved in jaw development, which makes sense, given the range of dietary adaptations seen in cichlids. Thus, one mechanism that has hastened cichlid speciation is intense selection pressure acting on many genes.
But individual genes can have great power, too. My laboratory has found evidence that a single gene determines the orientation of the stripes on the cichlids. Genes that on their own make a huge difference in an organism's appearance, as the stripe coders do, could help explain why there are so many kinds of cichlids.
We were also eager to survey the cichlid genomes for multiple copies of individual genes. Scientists have known for decades that duplication of genes—which arise from errors in DNA replication—is one of the most important mechanisms by which gene function can rapidly diversify. In essence, if a gene were duplicated, the new copy might be free to change without denying the animal any of the material encoded by the gene (because the other copy would still work), and the change could potentially help the creature to adapt to its environment. Typically genes are quite constrained in the mutations that can occur without harming the “host.” Our genome analyses show that cichlids have rates of gene duplication that are up to five times higher than that of “normal” fishes such as the sticklebacks.
A third type of genomic mechanism we sought to analyze was the activity of so-called jumping genes. Around 16 to 19 percent of the genome of a typical fish is made up of such DNA sequences, which do not serve an obvious function but make copies of themselves and jump from one location in the genome to another. They can be a force of evolution if they insert themselves close enough to a protein-coding gene to change its function. In the cichlid genomes, we have found telltale signs of several periods in which jumping genes accumulated rapidly, including one that coincided with the Lake Victoria radiation. The timing suggests that jumping genes may have helped facilitate the diversification of cichlids during such events.
We also examined DNA sequences that ordinarily do not change much. Certain regions of the genome that do not specify amino acids in proteins tend to be highly conserved across large evolutionary time spans. These conserved noncoding elements (CNEs), as they are known, probably affect the functioning of genes. Otherwise, random mutations would accumulate, as they typically do over time in nonconserved regions, rendering these regions different from species to species. Cichlids share a number of CNEs with one another and with more distantly related species, such as sticklebacks. But when we took a closer look at the DNA, we found that although the cichlid CNEs are similar enough from species to species to spot them, they have changed more than one would expect for CNEs. Our cichlid genome comparisons found that about 60 percent of the CNEs had undergone significant changes in particular lineages of cichlids. This surprisingly high percentage suggested that the genes to which these CNEs are linked might have undergone a change in function. Subsequent experiments bore this hunch out: researchers assessed the function of conserved and altered cichlid CNEs by inserting this genetic material into the genomes of zebra fish and found that the altered CNEs switched on their associated genes differently than the unaltered CNEs did—a sure sign that evolution of the CNEs led to changes in gene function in cichlids.
Click or tap to enlarge

Credit: Jen Christiansen
Another kind of genetic material that tends to be highly conserved across species is microRNA. MicroRNAs are small molecules that act as switches for genes, telling them where and when they should do their work. We were surprised to find 40 that had never been seen before in other fishes. We then studied cichlid embryos to see where in the body some of these microRNAs were regulating gene activity. It turns out they work in a highly specific manner, influencing genes only in certain tissues, such as a particular region of the facial skeleton. The targeted activity of microRNAs hints that they could have enabled the kind of precision sculpting that gave rise to the various feeding specializations cichlids possess, among other traits.
We remain a long way from fully understanding if and how all the hundreds of microRNAs in cichlid genomes actually foster evolutionary change, but they are strong candidates for such a role. We hypothesize that by preventing genes from being switched on at the wrong place or time, microRNAs could encourage both more variation and more precision in orchestrating the intricate ballet of genes that interact to make slightly different teeth, jawbones, color patterns, courtship behaviors, and so on—variation that is the basis for adaptation and speciation.
What’s old is new
This initial foray into cichlid genomes suggests that new random mutations, such as those seen in the CNEs and those that give rise to novel microRNAs, have figured significantly in the extraordinary evolution of these fishes. But we suspect that relatively old genetic variation, including that from duplicated genes and jumping genes, may have done most of the work. These variants lurked quietly in the genome until new ecological opportunities—for instance, those that arose when ancestral river-dwelling cichlids colonized the Great Lakes of Africa—suddenly made them advantageous. By tapping into that ancient genetic variation, natural selection created species suited to the new habitats.
We think old variation is the key because when we look at the genomes of these cichlid species, we cannot find many fixed genetic differences between them. That is, there are very few examples of genes for which all members of a species carry the same variant. Instead the gene pool of a species retains old gene variants even after fish have branched off from their ancestors to form a new species. And not only does a young species retain old DNA from its ancestors, but it may still be similar enough to interbreed and hybridize with closely related species. Such mixing would allow new gene variants to flow across species boundaries—more potentially useful genetic material that can be recycled when needed. The retention of old genetic variation, in addition to fueling rapid diversification in cichlids, can also help explain how the same ultraspecific traits have evolved over and over in separate lineages: we suspect that traits such as asymmetrical jaws and Angelina Jolie lips may not have arisen anew each time; rather the same genes and gene switches were repeatedly recruited into service. This hypothesis awaits testing.
The genomic mechanisms described here were not the only drivers of cichlid evolution. Surely environmental factors had a crucial part in establishing the patterns and rates of diversification in this group. Differences in the extent of cichlid diversity in the various radiations of these fishes around the world support this surmise: in Africa and in Nicaragua, the radiations that occur in lakes with more complex habitats (and thus more ecological niches) have more species than the radiations in lakes with simpler habitats. In addition to the speciation that occurred as cichlids evolved feeding specializations to fill these niches, further diversification took place as skin color differences arose and females developed preferences for particular hues.
We still have much to learn. Now that we have complete genomes, as well as powerful new techniques for analyzing them, though, our knowledge will advance very quickly indeed. I expect that the mechanisms underlying the speed of cichlid speciation will continue to be an area of intense research. Soon we will have a far deeper understanding of the language of the genome and the DNA that connects all living things even as it drives them apart.