When it comes to understanding the intricate world of chemistry, one of the fascinating phenomena that captures the attention of scientists and researchers is nucleation. Nucleation, the process by which a new phase or substance forms from a pre-existing one, is a fundamental concept in chemistry and plays a crucial role in various natural and industrial processes.
In this article, we will explore nine surprising facts about nucleation that will leave you amazed. From the spontaneous creation of crystals to the influence of impurities on nucleation, these facts will shed light on the remarkable intricacies of this phenomenon. So, buckle up and prepare to embark on a journey to unravel the mysteries of nucleation and uncover the surprising insights that lie beneath its surface.
Key Takeaways:
- Nucleation is the cool science behind the formation of bubbles in soda, snowflakes, and even clouds! It’s like the magic ingredient that makes these things happen in nature and in our drinks.
- Scientists can use nucleation to make materials stronger and better. It’s like a secret recipe for creating super materials with special powers, like making them extra tough or extra durable.
Nucleation is the formation of a new phase.
When a system undergoes a phase transition, nucleation is the process by which a new phase starts to form. This can be observed in the formation of ice crystals in supercooled water or the condensation of vapor into droplets.
Nucleation is essential for the formation of clouds.
Clouds are formed when water vapor in the atmosphere condenses onto tiny particles, called cloud condensation nuclei. These nuclei act as sites for nucleation, allowing water droplets to form and ultimately creating clouds.
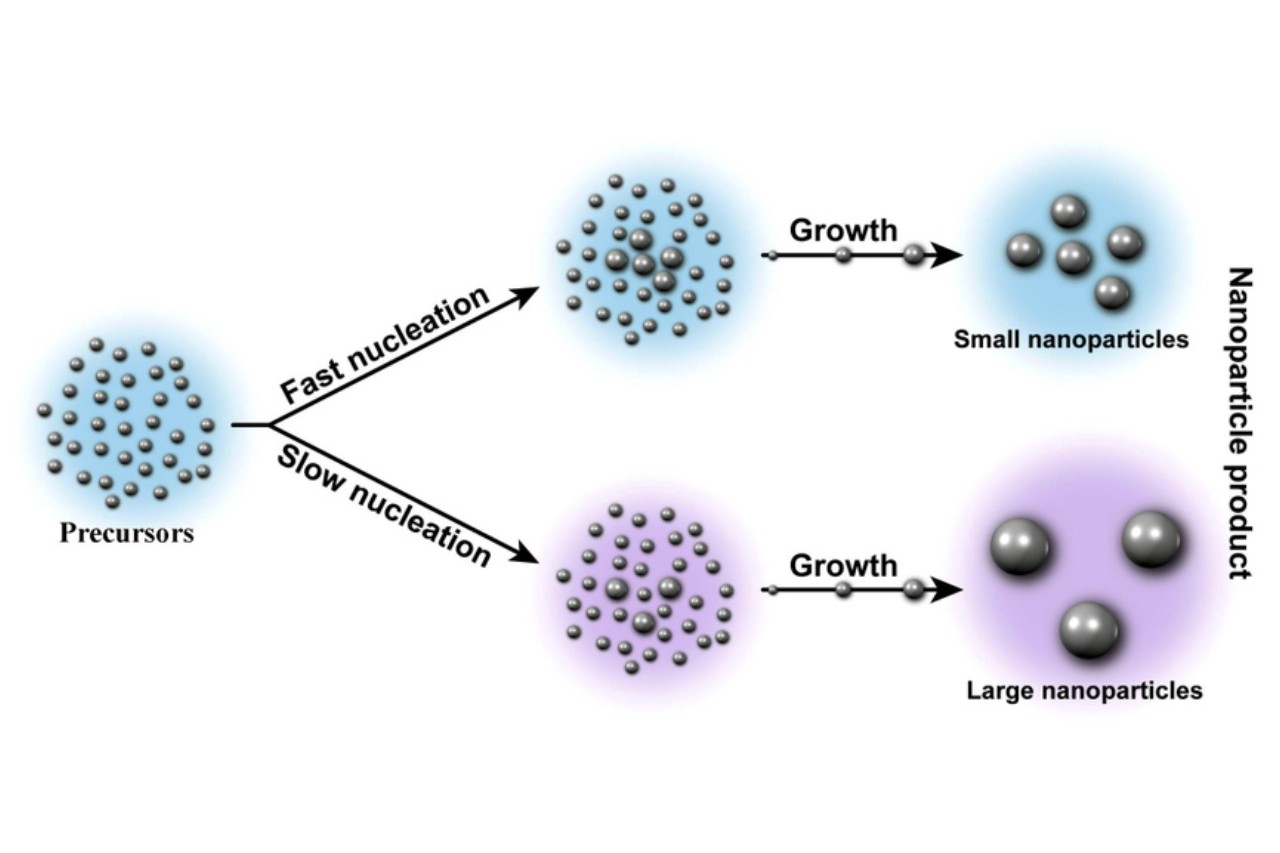
Nucleation is crucial in crystal growth.
In the formation of solid crystals, nucleation plays a key role in determining the size and structure of the crystals. The rate of nucleation affects how quickly and uniformly the crystals grow, influencing their properties and applications.
Nucleation can be homogeneous or heterogeneous.
In homogeneous nucleation, the formation of the new phase occurs uniformly throughout the system. On the other hand, heterogeneous nucleation involves the presence of external surfaces or impurities that act as nucleation sites, accelerating the process.
Nucleation is influenced by temperature and pressure.
The rate of nucleation is highly dependent on temperature and pressure conditions. Generally, an increase in temperature or a decrease in pressure promotes nucleation and vice versa.
Nucleation is a key step in the formation of bubbles in carbonated drinks.
Ever wondered why bubbles form when you pour a carbonated drink? It’s due to nucleation! The dissolved carbon dioxide gas in the drink forms bubbles on the surface of impurities, such as dust particles or imperfections in the glass.
Nucleation is involved in the formation of snowflakes.
Snowflakes have intricate and unique patterns, thanks to the process of nucleation. As water vapor freezes in the cold atmosphere, it undergoes nucleation, leading to the formation of ice crystals that eventually grow into beautiful snowflakes.
Nucleation is a critical step in the formation of protein crystals.
Protein crystals are essential for studying the structure and function of proteins. Nucleation initiates the formation of these crystals, which are crucial in fields such as biochemistry and drug development.
Nucleation can be controlled to improve material properties.
Scientists and engineers harness the principles of nucleation to control the formation of materials with desired properties. By manipulating nucleation conditions, they can enhance the strength, durability, and other characteristics of various materials.
In conclusion, these 9 surprising facts about nucleation shed light on the remarkable role it plays in a wide range of natural and artificial processes. Understanding nucleation can lead to advancements in various fields and applications, making it a subject of great scientific interest.
Conclusion
In conclusion, nucleation is a fascinating and complex process that plays a crucial role in various scientific fields, from chemistry to materials science. The nine surprising facts about nucleation highlight the remarkable intricacies of this phenomenon.From the unexpected influence of impurities to the connection between nucleation and crystal growth, each fact sheds light on different aspects of nucleation. The role of supersaturation and the impact of temperature on nucleation are also intriguing factors to consider.Understanding nucleation is not only important for academic research, but also has practical applications in industries such as pharmaceuticals, agriculture, and even food production.By delving into the surprising facts about nucleation, scientists can continue to unravel the mysteries of this process and uncover new possibilities for advancements in various scientific disciplines.
FAQs
Q: What is nucleation?
A: Nucleation is the process in which atoms, ions, or molecules come together to form a stable nucleus, initiating the formation of a new phase, such as the solidification of a liquid or the formation of a crystal.
Q: What factors can influence nucleation?
A: Factors such as temperature, pressure, concentration, and the presence of impurities can all affect nucleation. Higher supersaturation levels increase the likelihood of nucleation, while impurities can either inhibit or enhance the process.
Q: How does nucleation relate to crystal growth?
A: Nucleation is the initial step in crystal growth. Once a stable nucleus forms, additional atoms, ions, or molecules can adhere to it, leading to the growth of a larger, organized crystal structure.
Q: Can nucleation occur in non-chemical processes?
A: Yes, nucleation is not limited to chemical processes. It can also occur in atmospheric conditions, such as the formation of clouds or the crystallization of snowflakes.
Q: Are there any practical applications of nucleation?
A: Yes, nucleation has numerous practical applications. It is used in pharmaceuticals to control the formation of crystals and improve drug solubility. It is also essential in agriculture for the formation of ice crystals in frost protection systems and in food production for the controlled crystallization of chocolate.
Q: Can nucleation be controlled or manipulated?
A: Yes, researchers are continuously exploring ways to control and manipulate nucleation. By understanding the underlying principles, it is possible to optimize conditions and promote or inhibit nucleation according to specific needs.
Q: Can nucleation be studied experimentally?
A: Yes, nucleation can be studied experimentally through various techniques, including microscopy, spectroscopy, and thermodynamic measurements. These methods allow scientists to observe and analyze nucleation processes in detail.
Q: Is nucleation only relevant in scientific research?
A: No, nucleation is also relevant in everyday life. Understanding nucleation phenomena helps in various fields, such as weather forecasting, agriculture, and the development of new materials.
Q: Are there any ongoing challenges in the study of nucleation?
A: Yes, nucleation remains a complex and challenging subject of study. The processes involved are often intricate and difficult to observe directly, requiring advanced experimental techniques and theoretical modeling to gain deeper insights.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
