Introduction
Over the last 30 years, there has been an increase in the number of antiepileptic drugs (AEDs) available for treating patients with seizures. There are more than 25 AEDs in the market that have led to enhanced treatment for many; the surge in medications, neurostimulation devices, surgical options, dietary therapies, and nonpharmacologic adjunctive interventions, has made decision making more complex. As options continue to increase it is critical for all neurologists, not just epileptologists, to be acquainted with the existing tools in our arsenal. In this article, we focus on the proper selection of pharmacologic options for our patients.
There is no set algorithm for prescribing AEDs and there are few examples of head-to-head comparison trials to guide our choice of pharmacologic treatment. Most drugs are initially approved as adjunctive, or add-on, agents. This translates to less efficacy data for drugs as monotherapy, particularly in patients who are recently diagnosed or medication naïve. Understanding drug risks and benefits is key. It is important to understand that both medication- and patient-related factors influence appropriate drug selection. The goal is to provide the best chance for seizure freedom with the lowest risk for potential side effects (ie, tolerability).
Patient Factors
Patient Characteristics
Patient demographics including age, sex, and race should be considered. Prior to initiation with carbamazepine, patients of Asian descent should be tested for the HLA B*1502 allele, which is associated with increased risk of Stevens-Johnson syndrome and toxic epidermal necrolysis.1
Some studies show nonadherence rates around 30%,2 and patients who do not follow treatment regimens have worse seizure control, decreased quality of life, decreased productivity, and increased morbidity and mortality. It is prudent to counsel patients and understand what leads to nonadherence.
Treatment of women with epilepsy is a unique challenge as hormonal variations may affect seizure frequency, and there is always the concern of potential pregnancy in those of childbearing age. Besides choosing an appropriate AED for the underlying seizure disorder, teratogenic risk based on available data must be considered and discussed. When pregnancy is planned, setting and achieving target AED levels before pregnancy is ideal. Understanding how physiologic changes per trimester affect drug levels is crucial to monitoring and adjusting doses.3,4 The interaction of AEDs and oral contraceptives is a frequent concern to discuss with the patient during AED selection(See Reproductive Issues and Epilepsy also in this issue).
It is also important to understand the hormonal nature of catamenial seizures. For patients with regular menses there are 3 distinct patterns: perimenstrual epilepsy (C1), at ovulation (C2), and during luteal phase (C3).5 Understanding which pattern a patient has will aid in timing increased medication dosing, additional AED need, or hormonal therapy during the menstrual cycle. Common treatments for catamenial seizures include progesterone supplementation at days 14-28. However, a randomized double-blind placebo-controlled trial determined there was no difference between progesterone and placebo for patients with either catamenial or noncatamenial seizures. Data suggested the level of perimenstrual seizure exacerbation is a significant predictor of response to progesterone therapy, and that there may be a subset of women who may experience significant benefit from progesterone therapy.6
Seizure Characteristics
The treatment initiation decision is dictated by the circumstances of a patient’s seizure event(s) (eg, provoked vs unprovoked, single vs repeated episodes). In 2015 the American Academy of Neurology (AAN) and American Epilepsy Society (AES) released an evidence-based practice guideline for managing adult patients after a first unprovoked seizure. The risk of seizure recurrence is greater in the first 2 years; clinical variables such as a brain insult or EEG abnormality increase this risk. Immediate AED therapy is likely to reduce recurrence risk within the first 2 years compared to delayed treatment.7 When choosing an appropriate AED to initiate (Table 1), it is paramount to understand the type of disorder being treated and if an underlying epilepsy syndrome exists. Seizure medications are often divided into narrow spectrum medications that are useful in treating focal onset seizures or broad spectrum that are useful in treating both focal onset and generalized onset seizures. Initiating treatment with a narrow spectrum agent in a patient with generalized onset seizures could lead to seizure worsening or pseudoexacerbation.
Medication Factors
Various pharmacodynamic and pharmacokinetic parameters should be considered, including the mechanism of action (MOA), bioavailability, protein binding, half-life, drug-drug interactions, and elimination (metabolism and/or excretion) (Table 1). Another key element to consider is the side effect profile of each AED. These factors can lead to poor tolerability, decreased adherence, increased morbidity, and worsened seizure control, which may increase a patient’s risk of death. Drowsiness and cognitive impairment are common side effects of most AEDs although the degree of side effects varies among medication classes. Topiramate is more often associated with cognitive side effects, that in pairwise comparison are significantly worse than side effects of carbamazepine, lamotrigine, levetiracetam, oxcarbazepine, phenytoin, and valproic acid (Table 1).10,11 Side effects can be additive as more AEDs are taken. If a person has baseline cognitive impairment or would encounter a negative impact on daily functioning or employment, these types of side effects should be kept in mind.
Ease of administration can greatly affect patient adherence to treatment. Reducing frequency of taking medication each day through the use of an extended release formulation should be considered when possible. Certain AEDs are available in liquid solution or IV formulation for patients with dysphagia. Cost is a crucial consideration. Many AEDs are available in generic formulations that can be considered first, if medically appropriate, and this should be addressed when there are prohibitive financial constraints. Although overestimated, there are a few AEDs with a narrow therapeutic index (NTI) for which small fluctuations in drug levels found in generic formulations may lead to lost efficacy at low doses or to side effects at high doses. Only phenytoin and carbamazepine are classified as NTI AEDs by the FDA. There are patient assistance programs and discount cards for many medications that can be provided as well.
Combining Drugs and Rational Polypharmacy
Combination therapy should be considered if monotherapy fails. Before prescribing combination therapy, it is important to understand different AED mechanisms of action. The goal of combination therapy is to maximize efficacy and minimize adverse effects, termed rational polypharmacy. A key concept is that the more medications a patient is taking, the greater risk she or he has for toxicity and side effects.12 A good rule of thumb is to use an AED that could treat a comorbidity (eg, headaches or a mood disorder) whenever possible, to limit polypharmacy.
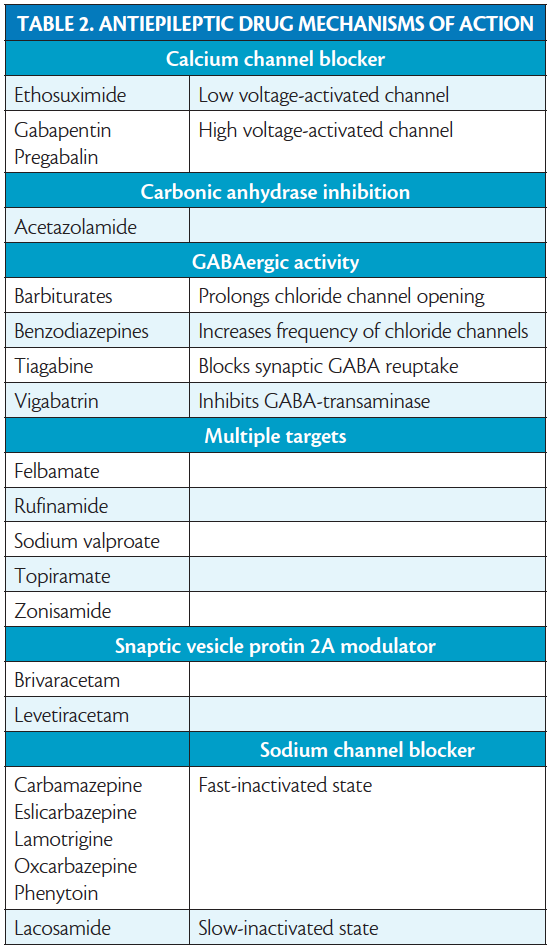
The AED mechanisms of action to keep in mind are shown in Table 2. Experimental models have shown that using AEDs with different mechanisms of action provides superior efficacy of combination therapy.13 Similarly there may be decreased efficacy when drugs with similar mechanisms of actions are combined (eg, phenytoin and carbamazepine.)14 Clinical studies show that combining drugs that block voltage-dependent sodium channels is more likely to produce side effects including dizziness, diplopia, and ataxia.15 The best supportive clinical evidence for synergism is seen when combining a drug that affects sodium channels with a drug that has multiple mechanisms of action, specifically lamotrigine and valproate.16
AEDs and Special Populations
Certain patient groups have unique considerations including avoidance of certain medication classes, dose adjustments, and cognizance of the effect epilepsy and certain AEDS have on any comorbid condition. All comorbid conditions should be considered when choosing medications for a specific patient.
Patients Over Age 60
In patients over age 60 with new-onset epilepsy, focal seizures are the most common presentation.17 Overall head-to-head efficacy and tolerability trials in this population are limited. Often a symptomatic or structural etiology is identified including stroke, a neurodegenerative process, or a tumor. Age-related changes in metabolism, renal clearance, gastrointestinal absorption, and serum albumin concentration may make dose adjustments necessary. For patients over age 60, it is often recommended medication be started at a lower dose and titrated slowly. It is also advised to avoid agents that induce hepatic enzymes (eg, phenytoin, phenobarbital, or carbamazepine) because hepatic-enzyme induction may worsen cardiovascular risk factors. A thorough investigation of possible medication interactions is necessary, as polypharmacy in this patient population is common. Another important point to consider is the risk of hyponatremia with oxcarbazepine, carbamazepine, or eslicarbazepine use, especially in those using diuretics.
Psychiatric Comorbidities
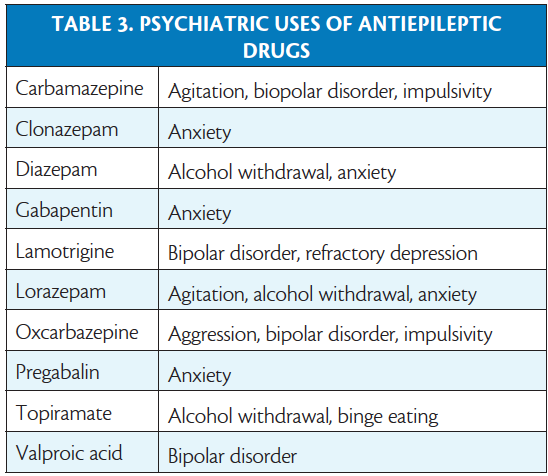
Several antiepileptic drugs are also used to treat psychiatric conditions and choosing a medication that may treat both the neurologic and psychiatric conditions may be beneficial (Table 3). In contrast, there are antiepileptic drugs with psychiatric side effects to be avoided in patients with certain psychiatric disorders. The cognitive slowing, fatigue, and somnolence associated with barbiturates, benzodiazepines, valproic acid, topiramate, gabapentin, and pregabalin may affect patients with various psychiatric comorbidities poorly. Levetiracetam is associated with mood swings, depression, and irritability and may exacerbate these symptoms. Perampanel has a black-box warning for severe psychiatric and behavioral reactions including aggression, hostility, and homicidal ideation.
Liver or Kidney Disease
In patients with hepatic impairment, AEDs that are metabolized in the liver (eg, benzodiazepines, carbamazepine, felbamate, phenytoin, phenobarbital, primidone, rufinamide, and valproic acid) are often avoided or require dose adjustments; in patients with kidney disease, the same is true for medications cleared renally (eg, gabapentin, pregablin, levetiracetam, eslicarbazepine, lacosamide, felbamate, and topiramate).19 In patients with renal failure, medications that are renally cleared are often avoided or require dose adjustments. For patients who undergo dialysis, careful attention is required because certain antiepileptic drugs are highly dialyzable including ethosuximide, gabapentin, lacosamide, levetiracetam, pregabalin, and topiramate. Dose supplementation at the time of dialysis may be needed for these drugs.19
HIV infection
Patients with HIV/AIDS are at risk for many central nervous system (CNS) conditions that can cause seizures (eg, toxoplasmosis, cryptococcal meningitis, CNS tuberculosis, progressive multifocal leukoencephalopahy, HIV encephalopathy, and CNS lymphoma).18 These conditions need to be treated in addition to using AEDs for seizure control. Many antibiotics, antifungals, and antiparasitics can lower seizure threshold so this should be considered as well. There are well known interactions between AEDs and antiretroviral drugs, including significant interactions between phenytoin and lopinavir, valproate and zidovudine, and ritonavir/atazanavir and lamotrigine.20 These may increase risk of antiretroviral failure and higher viral load. In these situations, selecting an AED with minimal drug-drug interactions (Table 1) is beneficial.
Malignancies
Certain chemotherapeutic drugs may lower seizure threshold (eg, L-asparaginase, busulfan, carmustine, cisplatin, cyclosporine, etoposide, and methotrexate).21 Patients taking these medications are at risk for direct CNS involvement by tumor, metabolic derangements, cerebrovascular complications, cerebral edema, and paraneoplastic processes. Selecting AEDs with minimal interactions is beneficial (eg, levetiracetam, lacosamide, briviact, lamotrigine, topiramate, zonisamide, pregablin, gabapentin ) (see Table 1).
Refractory Epilepsy and New Treatment Options
Approximately 30% of patients have refractory epilepsy despite best medication management. Refractory epilepsy is defined as the presence of seizures despite trials of at least 2 appropriately selected AEDs at tolerated therapeutic doses.22 Because these patients have significant morbidity and mortality,23 there has been significant interest in finding new options. There is growing awareness of cannabis-based therapies from physicians and patients alike that are a subject of significant investigation24,25; these are addressed in the article Cannabis, Cannabinoids, and Epilepsy in this issue.
Nonpharmacologic Adjunctive Interventions
Along with appropriate choice of AED, it important to keep in mind several nonpharmacologic adjunctive strategies that are useful in managing epilepsy. Patients should be counseled regarding seizures triggers, including sleep deprivation, stress, and mood disorders.4 In addition to medical treatment for comorbid mood disorders, relaxation techniques and referral to psychotherapy are often useful. This may be especially important for patients who have comorbid psychogenic nonepileptic spells that can benefit from cognitive behavioral therapy (CBT). Although data on the effect of alcohol on seizures is limited, patients with epilepsy commonly report alcohol as a trigger, and this should be discussed as well. Several studies suggest that treatment of obstructive sleep apnea in patients with epilepsy can lead to improved seizure control, specifically with the use of positive airway pressure therapy.26
Conclusion
There is no proven algorithm for selecting an AED for patients with epilepsy, and multiple factors must be considered during the selection process, including the type of epilepsy being treated (ie, focal vs generalized, specific syndrome, or if a clear first-line treatment exists), patient factors that narrow possible treatment options (ie, comorbid medical problems, concurrent medications, pregnancy, financial constraints), medication factors (ie, tolerability and rational polypharmacy), and nonpharmacological adjuncts. Unfortunately, there is a large proportion of patients who remain resistant to appropriate pharmacological therapy, despite a physician’s educated management. For those with refractory epilepsy, early referral to an epilepsy specialist and ideally a National Association of Epilepsy Centers (NAEC) Level 4 center, where alternative therapies such as advanced surgical treatments, neurostimulation, and research trials can be considered is prudent.
1 Chen P, Lin JJ, Lu CS, et al. Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N Engl J Med. 2011 Mar 24;364(12):1126-1133.
2. Hovinga CA, Asato MR, Manjunath R, et al. Association of non-adherence to antiepileptic drugs and seizures, quality of life, and productivity: survey of patients with epilepsy and physicians. Epilepsy Behav 2008;13(2):316-322.
3. Gerard EE, Meador KJ. Managing epilepsy in women. Continuum. 2016;22(1):204-226.
4. Husain A (Ed.) Practical Epilepsy. New York City, NY: Demos Medical Publishing: 2015.
5. Navis A, Harden C. A Treatment approach to catamenial epilepsy. Curr Treat Options Neurol 2016;18(7):30.
6. Herzog AG Progesterone vs placebo therapy for women with epilepsy. A randomized clinical trial. Neurology. 2012;78:1958-1966
7. Krumholz A, Wiebe S, Gronseth GS, et al. Evidence-based guideline: management of an unprovoked first seizure in adults: report of the Guideline Development Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2015 Apr 21;84(16):1705-13.
8. Zangaladze A, Skidmore C. Lacosamide use in refractory idiopathic primary generalized epilepsy. Epilepsy Behav. 2012;23(1):79-80.
9. Afra P, Adamolekun B. Lacosamide treatment of juvenile myoclonic epilepsy. Seizure. 2012;21(3):202-204.
10. Arif H, Buchsbaum R, Weintraub D, et al. Patient-reported cognitive side effects of antiepileptic drugs: predictors and comparison of all commonly used antiepileptic drugs. Epilepsy Behav 2009;14(1):202-209.
11. Abou-Khalil BW. Antiepileptic drugs. Continuum. 2016;22(1):132-156.
12. Brodie MJ, Sills GJ. Combining antiepileptic drugs--rational polytherapy? Seizure. 2011;20(5):369-375.
13. Bourgeois BF. Antiepileptic drug combinations and experimental background: the case of phenobarbital and phenytoin. Naunyn Schmiedebergs Arch Pharmacol. 1986;333(4):406-411.
14. Morris JC, Dodson WE, Hatlelid JM, et al. Phenytoin and carbamazepine, alone and in combination: anticonvulsant and neurotoxic effects. Neurology. 1987;37(7):1111-1118.
15. Besag FM, Berry DJ, Pool F, et al. Carbamazepine toxicity with lamotrigine: pharmacokinetic or pharmacodynamic interaction? Epilepsia. 1998;39(2):183-187.
16. Brodie MJ, Yuen AW. Lamotrigine substitution study: evidence for synergism with sodium valproate? 105 Study Group. Epilepsy Res. 1997;26(3):423-432.
17. Ramsay RE, Rowan AJ, Pryor FM. Special considerations in treating the elderly patient with epilepsy. Neurology. 2004;62(5):S24-S29.
18. Carlson C, Anderson CT. Special issues in epilepsy: the elderly, the immunocompromised, and bone health. Continuum. 2016;22(1):246-261.
19. Biller J, Ferro JM. (Eds) Handbook of Clinical Neurology: Elsevier, Philadelphia, PA, 2014.
20. Krikorian S, Rudorf, DC. Drug-drug interactions and HIV therapy: what should pharmacists know? J Pharm Pract. 2005;18(4):278-294.
21. Nolan CP, DeAngelis LM. Neurologic complications of chemotherapy and radiation therapy. Continuum. 2015;21(2):429-451.
22. Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010;51(6):1069-1077.
23. Ryvlin P, Cucherat M, Rheims S. Risk of sudden unexpected death in epilepsy in patients given adjunctive antiepileptic treatment for refractory seizures: a meta-analysis of placebo-controlled randomised trials. Lancet Neurol. 2011;10(11):961-968.
24. FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy [press release]. Silver Spring, MD: US Food and Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm611046.htm Published June 25, 2018. Accessed August 18, 2018.
25. O’Connell BK, Gloss D, Devinsky O. Cannabinoids in treatment-resistant epilepsy: A review. Epilepsy Behav. 2017;70(Pt B):341-348.
26. Pornsriniyom D, Kim H, Bena J, et al. Effect of positive airway pressure therapy on seizure control in patients with epilepsy and obstructive sleep apnea. Epilepsy Behav. 2014;37:270-275.
Disclosure
SA received honoraria for serving on a medical advisory board of Greenwich Biosciences and receives research support from Sunovion.