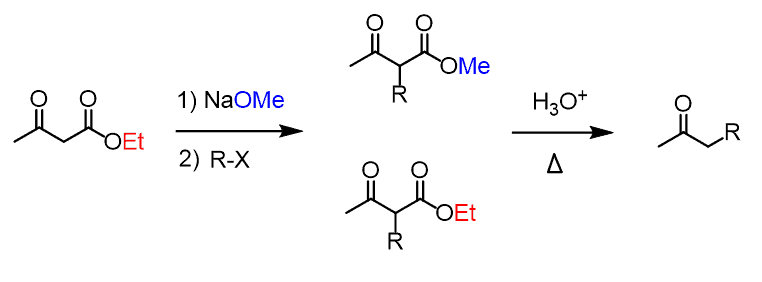
The acetoacetic ester synthesis is a useful synthetic tool for preparing ketones having one or two alkyl groups on the ɑ position:
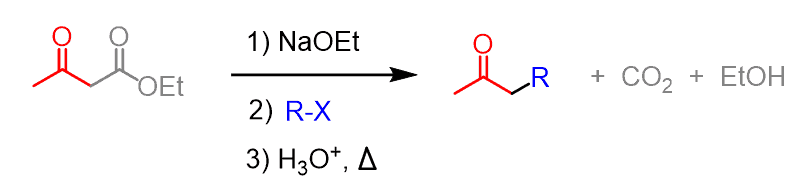
At first, this may look confusing since there is a whole ester group being lost in the course of the reaction. We will discuss what happens to the ester and how the reaction works in general, but let’s first put a summary diagram for predicting the outcome of the acetoacetic ester synthesis.
The net transformation here is the same as in the alkylation of ketones via formation of enolates.

The only purpose of the ester group is to make the ɑ hydrogen between the ketones more acidic. Remember, dicarbonyl compounds are about twice more acidic compared to their analogs with only one electron-withdrawing group:

Now, what is the benefit of having a more acidic ɑ position? Can’t we achieve this by a “simple” enolate alkylation and not have this ester group floating around?
The answer is yes, we can do that, however, having a more acidic ɑ hydrogen allows for using a weaker base and therefore, carrying out the reaction in milder conditions.
For example, alkylation of cyclohexanone requires using LDA, while acetoacetic ester can be deprotonated by sodium ethoxide:
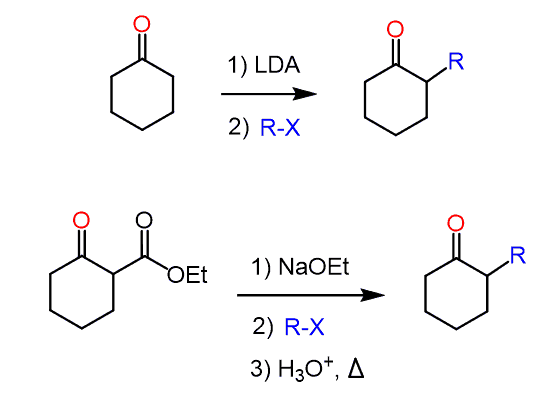
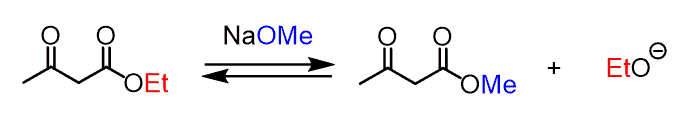
Careful, however, not to choose a base solely based on satisfying the pKa requirement! The alkoxide ion should have the same alkyl group as the ester of the starting β-keto ester.
For example, to deprotonate the ethyl acetoacetic ester, an ethoxide salt must be used. If a methoxide or a hydroxide is used, a problem of transesterification occurs:
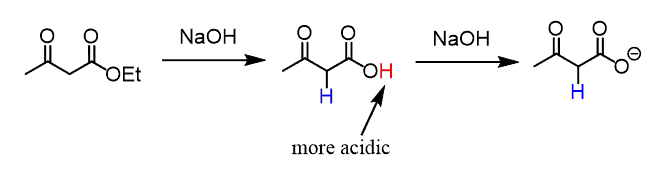
A hydroxide base, on the other hand, may hydrolyze the ester to a carboxylic salt before deprotonating the ɑ carbon:
Using an alkoxide of a different alkyl group wouldn’t matter in a long run since the ester group is cleaved off at the end anyway:
Decarboxylation in the Acetoacetic Ester Synthesis
There is one important question we haven’t addressed yet – what happens to the ester group? And here, what you need to remember is that esters having a carbonyl group on the β position (β-keto ester) undergo decarboxylation when heated in acidic conditions:
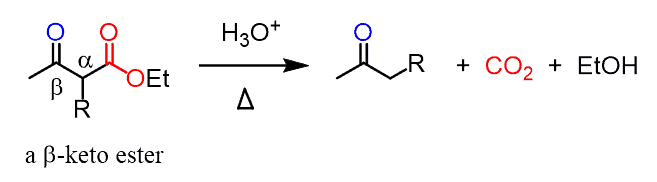
In the first part, the ester is hydrolyzed to a carboxylic acid which then loses carbon dioxide through a nicely arranged six-membered transaction state:

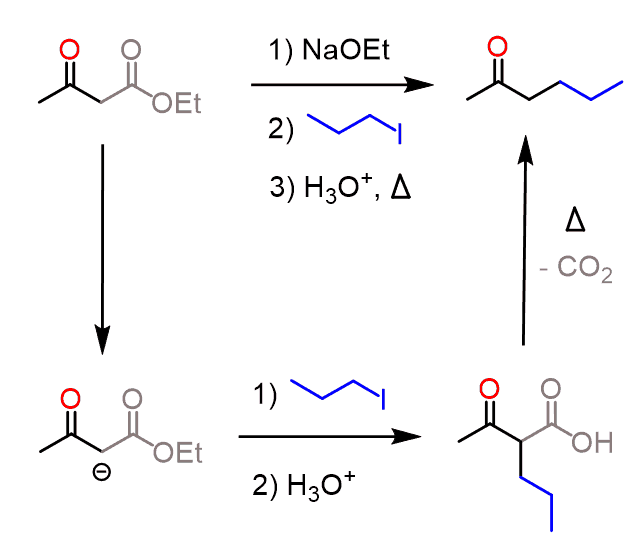
The resulting enolate tautomerizes to a ketone with a newly incorporated alkyl group on the ɑ position.
Breaking down this in steps, we can, for example, show the details of transforming acetoacetic ester into 2-hexanone:
As always, practice problems on the acetoacetic ester synthesis and alpha carbon chemistry can be found here:
Acetoacetic Ester Enolates Practice Problems
Enolates in Organic Synthesis – a Comprehensive Practice Problem
Check Also
- Alpha Halogenation of Enols and Enolates
- The Haloform and Iodoform Reactions
- Alpha Halogenation of Carboxylic Acids
- Alpha Halogenation of Enols and Enolates Practice Problems
- Aldol Reaction – Principles and Mechanism
- Aldol Condensation – Dehydration of Aldol Addition Product
- Intramolecular Aldol Reactions
- Aldol Addition and Condensation Reactions – Practice Problems
- Crossed Aldol And Directed Aldol Reactions
- Crossed Aldol Condensation Practice Problems
- Alkylation of Enolates Alpha Position
- Enolate Alkylation Practice Problems
- Acetoacetic Ester Synthesis
- Acetoacetic Ester Enolates Practice Problems
- Malonic Ester Synthesis
- Michael Reaction: The Conjugate Addition of Enolates
- Robinson Annulation, Shortcut, and Retrosynthesis
- Claisen Condensation
- Dieckmann condensation – An Intramolecular Claisen Reaction
- Crossed Claisen and Claisen Variation Reactions
- Claisen Condensation Practice Problems
- Stork Enamine Synthesis
- Enolates in Organic Synthesis – a Comprehensive Practice Problem
