- 1Department of Oncology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 3State Key Laboratory of Southwestern Chinese Medicine Resources, School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 4Hospital of Chengdu University of Traditional Chinese Medicine, School of Clinical Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 5Department of Gastroenterology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 6TCM Regulating Metabolic Diseases Key Laboratory of Sichuan Province, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 7Geriatric Department, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
In many studies, the extensive and significant anticancer activity of chelerythrine (CHE) was identified, which is the primary natural active compound in four traditional botanical drugs and can be applied as a promising treatment in various solid tumors. So this review aimed to summarize the anticancer capacities and the antitumor mechanism of CHE. The literature searches revolving around CHE have been carried out on PubMed, Web of Science, ScienceDirect, and MEDLINE databases. Increasing evidence indicates that CHE, as a benzophenanthridine alkaloid, exhibits its excellent anticancer activity as CHE can intervene in tumor progression and inhibit tumor growth in multiple ways, such as induction of cancer cell apoptosis, cell cycle arrest, prevention of tumor invasion and metastasis, autophagy-mediated cell death, bind selectively to telomeric G-quadruplex and strongly inhibit the telomerase activity through G-quadruplex stabilization, reactive oxygen species (ROS), mitogen-activated protein kinase (MAPK), and PKC. The role of CHE against diverse types of cancers has been investigated in many studies and has been identified as the main antitumor drug candidate in drug discovery programs. The current complex data suggest the potential value in clinical application and the future direction of CHE as a therapeutic drug in cancer. Furthermore, the limitations and the present problems are also highlighted in this review. Despite the unclearly delineated molecular targets of CHE, extensive research in this area provided continuously fresh data exploitable in the clinic while addressing the present requirement for further studies such as toxicological studies, combination medication, and the development of novel chemical methods or biomaterials to extend the effects of CHE or the development of its derivatives and analogs, contributing to the effective transformation of this underestimated anticancer drug into clinical practice. We believe that this review can provide support for the clinical application of a new anticancer drug in the future.
1 Introduction
Cancer is one of the primary causes of mortality worldwide and has killed nearly 9.6 million people in 2018 (Bray et al., 2018), leading to a heavy global economic burden and an impact on the quality of life. Over several decades, research on the diagnosis and treatment of cancer has rapidly developed, leading to significant advances in clinical practice, particularly in targeted cancer therapies and immunotherapy. However, cancer remains inevitable due to the increased probability of tumor recurrence, the lack of anticancer drugs, and the adverse effects and resistance to conventional treatments (Deng and Cassileth, 2013; Jermini et al., 2019).
Traditional herbal medicine is rich in bioactive ingredients and is widely used in clinical practice. Recently, herbal medicine extracts have played an increasingly important role in the discovery of available medicines for the prevention and treatment of cancer, with nearly 80% of the therapeutics for cancer therapy including natural products, derivatives/analogs, or imitation of natural products (Banik et al., 2019; Hsieh et al., 2015; Newman et al., 2003; Shanmugam et al., 2017; S. F.; Yang et al., 2013). Natural products (i.e., alkaloids, polyphenolic compounds, diterpenoids, flavonoids, and sesquiterpenes) present in various parts of plants possess immense anticancer potential (Bishayee and Sethi, 2016; Millimouno et al., 2014; S. F.; Yang et al., 2013). Among these natural products, alkaloids and polyphenols offer distinct advantages in cancer treatment (Newman and Cragg, 2016).
Based on a literature review, four herbaceous plants, Chelidonium majus L., Macleaya cordata (Willd.) R. Br., Sanguinaria canadensis L., and Zanthoxylum asiaticum (L.) Appelhans, Groppo & J.Wen, which are well known for their treatment of cancer, skin diseases, gastrointestinal parasites, liver cirrhosis, jaundice, and other difficult miscellaneous diseases, have a long history of usage, especially for treating cancer, possibly leading to a deep exploration of the effective anticancer active compounds based on the traditional use of these plants. Isoquinoline alkaloids are the common pharmacologically relevant substances of these plants (Croaker et al., 2016; Ni et al., 2016; Zielińska et al., 2018; Zeng et al., 2021). As a premier member, CHE is an alkaloid that is extracted mainly from the abovementioned four plants and possesses numerous biological properties, including anti-inflammatory (Niu et al., 2011), anticancer (Jana et al., 2017), antidiabetic (Zheng et al., 2015), antifungal (Wei et al., 2017; He et al., 2018), and anti-parasitic activities (Li et al., 2011), as well as the activity against ethanol-induced gastric ulcers (Li et al., 2014). Therefore, CHE can be used as one of the main representatives of the anticancer effect of these four traditional plants, combined with the needs of contemporary cancer drugs; it is worth further exploring the anticancer effect of CHE.
The antitumor activity of CHE has been demonstrated in in vitro experiments for cytotoxicity and growth delay against various tumor cell lines (Chmura et al., 2000). CHE induces apoptosis in cancer cells, suggesting that CHE is an anticancer agent (Malikova et al., 2006). A large number of studies have succeeded in identifying the anticancer activity of CHE in a variety of cancers, such as uveal melanoma (Kemény-Beke et al., 2006), leukemia (Vrba et al., 2008), non–small cell lung cancer (Gao et al., 2010), triple-negative breast cancer (Lin et al., 2017), prostate cancer (Malíková et al., 2006), liver cancer (Zhang et al., 2011), and renal cancer (Chen et al., 2016). The anticancer effect of CHE has been confirmed and has received considerable research attention; however, this underestimated anticancer drug is not used in clinical treatments, and it is worth discussing the reasons.
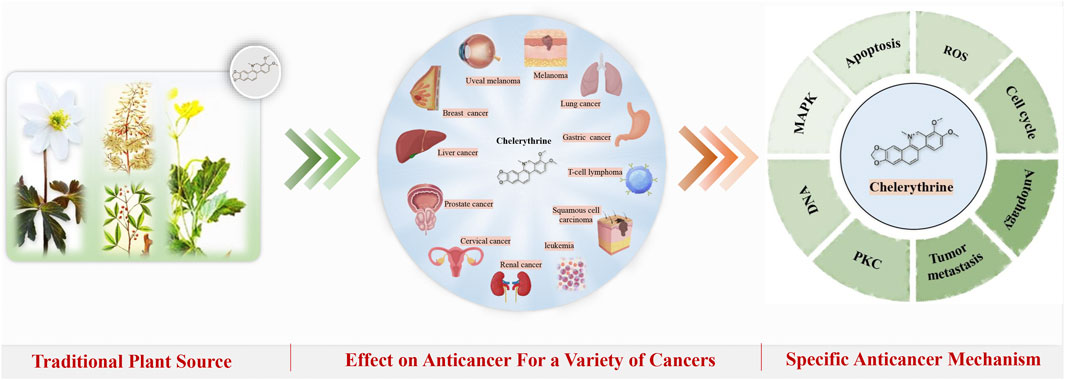
Although the four plants and their related compounds have certain anticancer activities that have been reported, there is still a lack of systematic reviews of CHE. This review article aimed to investigate and systematically cover all the findings on the molecular mechanisms of CHE in vitro and in vivo, especially the cancer-combating properties. Further studies are needed to explore the clinical application prospects of CHE and provide systemic evidence for the anticancer activity. (Figure 1).
2 Plant Sources and Structural Features of Chelerythrine
2.1 Plant Sources of Chelerythrine and the History of Usage
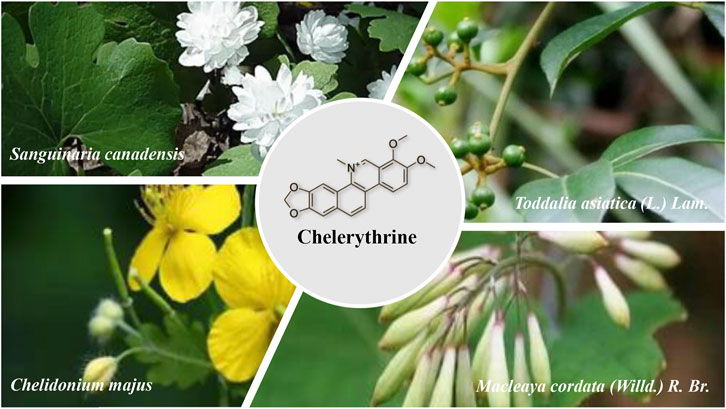
Chelerythrine (CHE) consists mainly of extracted and isolated alkaloid components from four plants of the families Papaveraceae (e.g., Chelidonium majus L., Macleaya cordata (Willd.) R.Br., and Sanguinaria canadensis L.) and Rutaceae (e.g., Zanthoxylum asiaticum (L.) Appelhans, Groppo & J.Wen) (Zhang et al., 2005; Malikova et al., 2006; Wang et al., 2013). Direct scientific studies have proven that the abovementioned four plants have antitumor medicinal values (Iwasaki et al., 2010; Deljanin et al., 2016; Ali et al., 2020; Tuzimski et al., 2021), and CHE, as the most representative active compound for these plants, has also undergone a large number of studies on anticancer activity in vitro and in vivo, so there is a clear link between the compound and the traditional uses and the effects of these plants (Chmura et al., 2000). (Figure 2)
2.1.1 Traditional Use of Chelidonium. majus L
CHE is one of the main effective compounds of the traditional herbal medicine Chelidonium. majus L. of the Papaveraceae family, which has a long history of clinical treatment. The plant C. majus, or greater celandine, is widely distributed in Asia, Europe, North Africa, and North America and is an admired plant with tremendous medicinal value that has been used in clinical treatment from antiquity (Gilca et al., 2010; Zielińska et al., 2018).
In folk medicine experience in Europe, C. majus is used to treat skin diseases in many areas, such as removing warts (Koleva et al., 2015), and C. majus has significant effects on skin wounds, scabies, ulcers, and skin eruptions (Mamedov et al., 2005; Jarić et al., 2007; Menkovi et al., 2011). The second widely applied medicinal value is for the treatment of liver diseases and jaundice, where C. majus can relieve precancerous lesions of liver cirrhosis and treat hepatitis and can be used for inflammation of the gallbladder and bile duct (Jarić et al., 2007; Savikin et al., 2013; Kujawska et al., 2016, 2017). Moreover, this herb has been used mostly to cure eye diseases since the Middle Ages, protecting against eye infections and sight impairment (Mayer et al., 2002; Kujawska et al., 2017). With the development of science, the medicinal value of this plant has been further explored, the anticancer activities of C. majus have also been investigated, and more verifiable scientific experiments have been conducted in vitro and in vivo in the traditional use of C. majus. In recent decades, numerous reports have proven the antitumor actions of C. majus, regardless of whether it is a plant extract of C. majus or a compound from this plant, manifesting efficacy in multiple cancer models with different molecular mechanisms (Biswas et al., 2008; Deljanin et al., 2016; Havelek et al., 2016; I et al., 2015; Park et al., 2015; Warowicka et al., 2019). In addition, several clinical trials have also demonstrated the beneficial effects of C. majus in the treatment of human cancer (Uglyanitsa et al., 2000; Gansauge et al., 2002; Ernst and Schmidt, 2005; Habermehl et al., 2006). More than 50 alkaloids have been isolated from this plant (Maji and Banerji, 2015), and the extracts and purified compounds exhibit a wide range of biological activity. The antitumor activity supports the clinical treatment of cancer, so the antitumor properties of this plant have been the primary focus (Mayer et al., 2002; Savikin et al., 2013). Major pharmacologically relevant components of C. majus, most of which are isoquinoline alkaloids, include berberine, chelerythrine, chelidonine, coptisine, and sanguinarine (Colombo and Bosisio, 1996). The antiproliferative, proapoptotic, and cytotoxic effects of berberine, chelerythrine, and sanguinarine on cancer cell lines of C. majus are based on their abilities (Zielińska et al., 2018). The anticancer activities of berberine and sanguinarine have been reviewed (Jin et al., 2016; Achkar et al., 2017), and it is necessary to dig deeper into CHE.
2.1.2 Traditional Use of Zanthoxylum asiaticum (L.) Appelhans, Groppo & J.Wen
CHE is also one of the most characteristic compounds for Zanthoxylum asiaticum (L.) Appelhans, Groppo & J.Wen (another name is Toddalia asiatica (L.) Lam.), a plant from the family Rutaceae that is distributed mainly in tropical Africa and southeastern Asia and the southern areas of China (Xia and Liu, 2007), where the whole plant has been used as a drug for hundreds of years. T. asiatica has been chronicled as a treatment for colds, rheumatism, analgesia, injury, and hemoptysis in China (Shi et al., 2011), while the plant has also been used in the treatment of malaria in traditional practice in Africa, where the other most frequent diseases were followed by cough, chest pain, and sore throat (Ngarivhume et al., 2015; Orwa et al., 2013, Orwa et al., 2008). Based on these traditional medicinal experiences, further modern pharmacological studies have found antimalarial, antibacterial (Duraipandiyan and Ignacimuthu, 2009; Raj et al., 2012), and antidiabetic activities (Irudayaraj et al., 2013). At the same time, the antitumor effect of T. asiatica (Iwasaki et al., 2010; Li et al., 2018) and its drug activity in the treatment of arthritis and osteoporosis were also found (Watanabe et al., 2015; K.; Yang et al., 2013). Consequently, T. asiatica has often been used in folk medicine to treat different diseases, including but not limited to injuries, arthritis, and tumors.
According to the “Chinese Materia Medica” issued by the State Administration of Traditional Chinese Medicine, T. asiatica is used to treat malignant sores and lumps with the efficacy of detoxification, which is closely related to treating cancer. Based on the traditional use of this plant to treat cancer, multiple modern experimental studies have proven its anticancer activity(He and N, 1998; Iwasaki et al., 2010; Li et al., 2018) and anti-inflammatory activity (Kumagai et al., 2018; Ni et al., 2020). In recent years, with in-depth research, the chemical components in T. asiatica have gradually been discovered to be mainly coumarins and alkaloids, and alkaloids in T. asiatica play an important role in antitumor effects, especially benzophenanthridine alkaloids. CHE is the main benzophenanthridine alkaloid in this plant (Zeng et al., 2021). Combined with traditional experiences and studies, the anticancer activity of T. asiatica is worthy of in-depth exploration, and due to the important antitumor effect derived from CHE, in this article, the anticancer effect of CHE is comprehensively expounded in recent years to provide a reference for the related research and application of this medicinal material.
2.1.3 Traditional Use of Macleaya cordata (Willd.) R. Br
Macleaya cordata (Willd.) R. Br. is an herbaceous plant that also belongs to the Papaveraceae family and is widely dispersed in China; the plant is called “Bo Luo Hui” and includes mainly alkaloids such as sanguinarine and CHE (Kosina et al., 2010). This traditional drug was used to relieve pain and has positive effects on the treatment of skin diseases, sores and ulcers, and rheumatism in folk experience (Lin et al., 2018). Modern studies have identified that the pharmacological actions of M. cordata can reduce inflammation and antibacterial and antiviral activities (Sedo et al., 2002; Ni et al., 2016), and M. cordata is extensively used in the treatment of skin diseases such as scabies and corporis (Luo, 1987), some gynecological diseases, pneumonia, bacterial infections(Wu and Zhu, 2002), expulsion of parasites, and cancer (Chen et al., 2020).
Meanwhile, similar to T. asiatica, according to the “Chinese Materia Medica” issued by the State Administration of Traditional Chinese Medicine, M. cordata was also effective in the treatment of malignant sores and lumps in traditional Chinese medicine. The current studies suggest that M. cordata has anticancer properties, and its purified compounds and/or crude extract possess antitumor activity (Lin et al., 2018). M. cordata extracts exhibited antitumor activity in cancer (Ali et al., 2020), and alkaloids extracted from M. cordata exhibit bioactivity and anticancer properties, as well as strongly inhibiting the proliferation of cancer cell lines, so these alkaloids are the main pharmacological evidence that M. cordata can be used to treat cancer (Liu et al., 2013; Si et al., 2020; Sai et al., 2021). Therefore, further pharmacological studies have revealed that alkaloids are the major bioactive components of M. cordata (Chen et al., 2009). To date, 147 alkaloids have been identified based on their chemical structures. Most of these compounds are isoquinoline alkaloids; furthermore, the two main components sanguinarine and chelerythrine are considered responsible for the pharmacological activity of M. cordata (Lin et al., 2018). Therefore, the anticancer effect of CHE is closely related to M. cordata.
2.1.4 Traditional Use of Sanguinaria canadensis L
Sanguinaria canadensis L. is native to eastern North America. As a member of the Papaveraceae, S. canadensis contains six quaternary benzophenanthridine alkaloids (QBAs) (Bambagiotti-Alberti et al., 1991), while CHE is the second most important alkaloid in S. canadensis. S. canadensis is a traditional medicinal plant in Europe and North America that is used for treating pain, vomiting, gastrointestinal bleeding, abdominal mass, hemorrhagic tuberculosis, wound infection, gangrene, asthma, pertussis, pneumonia, diphtheria, dysentery, functional dyspepsia, jaundice, and liver disease (Croaker et al., 2016).
S. canadensis has a long history of curing cancer in clinical use since the mid-19th century, and people have used a black salve derived from S. canadensis (Jellinek and Maloney, 2005; Lin et al., 2018) to cure skin cancer (Croaker et al., 2016). Basic research also confirmed the possibility of its application in the treatment of human cancer through the in vivo experiments involving the S. canadensis extracts (Tuzimski et al., 2021). The anticancer activity of sanguinarine, the main component of S. canadensis, against various human malignancies has been validated and reviewed (Achkar et al., 2017). However, as the second major component of S. canadensis, CHE also has a clear and broad anticancer effect and can also represent the traditional anticancer use of S. canadensis, which can be explored deeply.
Almost all four of these plants have the same natural active compounds and traditional uses with analgesic, antibacterial, anti-inflammatory, and detoxification functions, and the most important frequently used traditional application is the anticancer effect. Among these compounds, isoquinoline alkaloids are the main pharmacological components that are well known for their anticancer effect. The most important alkaloids found in these plants, such as sanguinarine and CHE, can represent traditional anticancer uses and have a clear and more pharmacologically evidence-based demonstration of anticancer potential, providing new anticancer strategies for clinical practice (Achkar et al., 2017). In recent years, an increasing number of studies have identified that CHE has a significant broad-spectrum anticancer effect but is underestimated. Many direct scientific studies and references have proven that CHE can be used as the most important pharmacological component of the abovementioned four plants for anticancer purposes. Therefore, based on the efficacy of these traditional plants, this article further summarizes the anticancer effect of CHE and identifies new strategies for the treatment of cancer.
2.2 Structural Features of Chelerythrine
CHE (C21H18NO4) is a natural benzophenanthridine alkaloid with the chemical name of 1,2-dimethoxy-12-methyl-[1,3]-benzodioxolo-[5,6-c] phenanthridinium, and the relative molar mass is 348.37. According to the pH range, CHE exists in two forms, “charged iminium” in the pH range 1–6 and “alkanolamine” form in the pH range 8.5–11, which transform into each other in an equilibrium reaction and have extensive biological and biochemical performance in vivo. The studies reveal that the iminium form of CHE has a high binding affinity to DNA, but the neutral alkanolamine form does not bind to DNA. However, in the context of cancer, the intra-tumoral pH is low due to the acidic environment, and the functionality of this compound can be better. (Basu et al., 2013). CHE interacts with a variety of proteins and DNA, and the charged iminium form binds more strongly to DNA/RNA, while the alkanolamine state has more binding affinity to bovine serum albumin (Bhuiya et al., 2016). The most active part of CHE is the carbon adjacent to the quaternary nitrogen, attacked by the nucleophiles, and responsible for this compound’s observed toxicity.
3 AntiCancer Strategy of Chelerythrine
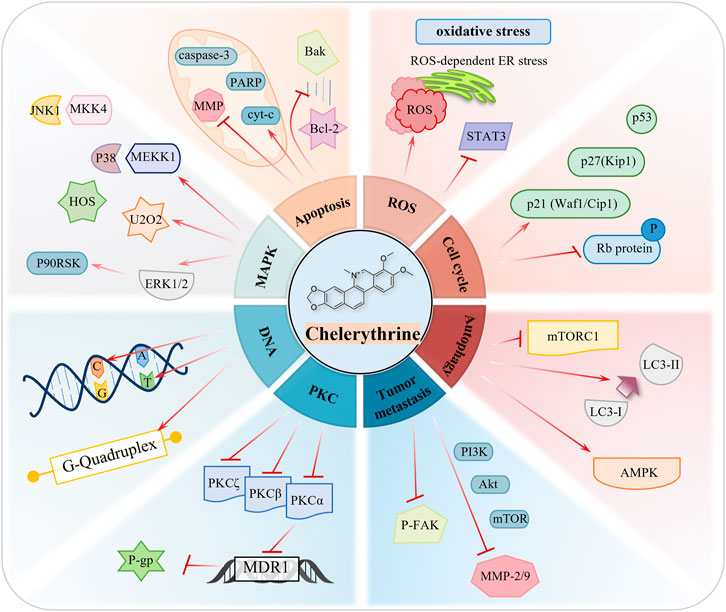
The anticancer targets and mechanisms of natural products have raised greater concern and have been widely reported throughout the years (Ren et al., 2019). Evidence based on multiple cancer experiments indicates that CHE is a broad-spectrum anticancer drug, which can be a prospective compound in the treatment of cancers. CHE causes the death of cancer cells through cell apoptosis, cell cycle arrest, autophagy, inhibition of invasion and metastasis, and generation of reactive oxygen species (ROS). (Figure 3; Table 1).
3.1 Inducing Cancer Cell Apoptosis
The human body eliminates damaged and aged cells to maintain homeostasis through a genetic apoptosis process under physiological conditions, while tumor cells evade apoptosis and grow indefinitely. Cells perform apoptosis programs through external or internal pathways. The external pathway is composed of ligands and death receptors that bind to Fas-related death domains (FADDs) and tumor necrosis factor receptor 1 (TNFR1)-related death domain (TRADD) interactions, which then form the death-inducing signal complex (DISC), assembly, activate caspase-8, and further initiate cell apoptosis (Wong, 2011). The intrinsic mitochondrial pathway is regulated by the Bcl-2 family. Bax and Bcl-2 bind to the mitochondrial membrane and contain proapoptotic or antiapoptotic proteins. Apoptotic bodies are formed by recruiting Apaf-1 and procaspase-9 to the cytoplasm, further triggering the downstream caspase-9/3 cascade reaction (Wong, 2011).
The anticancer ability of CHE is focused on cell apoptosis. Micromolar concentrations of CHE have been reported to significantly inhibit tumor cell growth, and this inhibitory effect is related mainly to the induction of apoptosis. In a radioresistant tumor model, CHE caused apoptotic cancer cell death by increasing sphingomyelinase activity and enhancing ionizing radiation (IR)–mediated cell killing (Chmura et al., 1997). Studies have found that CHE is capable of aggregating in mitochondria, which could cause mitochondrial dysfunction because of its cellular permeability and mitochondria-targeting ability (Lei et al., 2018). CHE has been used as an inhibitor of Bcl-2 family proteins (Herbert et al., 1990) and could significantly inhibit cell proliferation in tumor cells, inducing cell apoptosis by adjusting the expression of Bcl-2 family proteins and activating the pathway of mitochondrial apoptosis (Zhang et al., 2011). Chan et al. (2003) screened 12 species extracted from 107,423 natural products that can inhibit the binding of Bcl-xL and Baks, in which the four active ingredients were CHE. Therefore, further research confirmed that CHE is a functional inhibitor of Bcl-xL, bound at the BH region of Bcl-xL (Zhang et al., 2006), and was reported to interfere specifically with the interaction between Bcl-xL and Bax, which act directly on the mitochondria from tumor cells and lead to the release of Cyt-c. CHE treatment induces cancer cell apoptosis by activating caspase-3 and poly-ADP-ribose polymerase (PARP) and reducing Bcl-2 and mitochondrial membrane potential (MMP) (Zhang et al., 2012).
3.2 Inducing Reactive Oxygen Species-–Mediated Apoptosis
ROS are a typical byproduct of various cellular processes. As an important second messenger of signals, ROS can regulate cell death and cell proliferation (Covarrubias et al., 2008). High levels of ROS can activate a variety of proapoptotic signaling pathways, and cellular antioxidative machinery can balance the damaging effect of excessive ROS (Milkovic et al., 2019). Cancer cells typically obtain higher levels of ROS than normal cells to resist persistent internal oxidative stress (Cairns et al., 2011). Hence, targeting ROS and oxidative stress can be considered an excellent method to combat cancer cells.
Indeed, several studies have demonstrated that CHE induces ROS-mediated apoptosis in several types of cancer (Tang et al., 2017; Wu et al., 2018; He et al., 2019). CHE treatment can increase ROS generation in cancer cells, exceeding the internal antioxidant processing capacity and inducing cell death. A high level of ROS is also related to DNA and protein damage, which in turn, is related to the occurrence of apoptosis (Pelicano et al., 2004). In addition, CHE can mediate tumor cell death through ROS-dependent endoplasmic reticulum (ER) stress and STAT3 inactivation (Wu et al., 2018; He et al., 2019). Studies on five types of tumor cells, MCF-7, MDA-MB231, and HCT116, and one type of nontumor cell, AA-8, by Matkar et al. found that CHE induced a significant generation of ROS through the redox reaction cycle, especially hydrogen peroxide H2O2, which mediates rapid cell apoptosis (Matkar et al., 2008). The redox reaction cycle also consumes a large number of reducing coenzymes, which further accelerates cell apoptosis. Tumor cell necrosis and apoptosis were observed after treatment with CHE (Kemény-Beke et al., 2006; Vrba et al., 2008). Higher concentrations of ROS have a strong cytotoxic effect on cells, which indicates that CHE can promote not only cell apoptosis but also cell necrosis. CHE was shown to mediate the two-way death of tumor cells that produce a large amount of ROS in cells, which is one of the ways that CHE exerts antitumor activity.
3.3 Effect of Chelerythrine on Cell Cycle Arrest
Several cyclin-dependent kinases regulate the cell cycle that controls cell division and proliferation. Therefore, regulating cell cycle checkpoints that induce cell cycle arrest is a therapeutic target for cancer treatment (Otto and Sicinski, 2017). CHE was reported to have potent activity in cell cycle arrest, imparting anticancer effects on the human promyelocytic leukemia cell line by accumulating at the G1 phase and increasing at the G2/M phase when treated with a higher concentration (Vrba et al., 2008). Furthermore, when treating prostate cancer cell lines, CHE has a significant cytotoxic effect and induces cell cycle arrest by increasing cyclin kinase inhibitors p21Waf1/Cip1 and p27Kip1 (Malíková et al., 2006). CHE can also induce S-phase cell cycle arrest to inhibit cancer cell proliferation in gastric cancer (Zhang et al., 2012).
3.4 Inhibition of Tumor Invasion and Metastasis
Tumor metastasis is one of the causes of death in most cancer patients, especially due to the migration and invasion of cancer cells from the primary part and subsequent infiltration into foreign tissues (Valastyan and Weinberg, 2011). Therefore, blocking the metastatic tumor process with natural products would be efficient in preventing cancer metastasis. The expression and activation of the matrix metalloproteinases MMP-2 and MMP-9 are only increased in breast cancer patients (Scorilas et al., 2001), while MMP-2 induces cancer migration (Xu et al., 2005). CHE was found to downregulate the expressions of MMP-2 and MMP-9 through the PI3K/Akt/mTOR signaling pathway and change the cytoskeleton structure by reducing p-FAK, which inhibited the metastasis and invasion of cancer in a dose-dependent manner (Zhu et al., 2018).
3.5 Chelerythrine as an Effective Protein Kinase C Antagonist
Protein kinase C (PKC), which belongs to the serine/threonine kinase family and has multiple isoenzyme subtypes, was first discovered in the mouse brain in 1977 (Takai et al., 1977). PKC, closely related to the development of cancers and overexpressed in various tumor cells, can affect division and proliferation by catalyzing some small-molecule peptides and enzymes that bind to DNA in tumor cells.
CHE (IC50 = 0.66 μmol/L), a PKC inhibitor, not only binds to the catalytic domain and inhibits the expression of PKC (Herbert et al., 1990) but also affects the function of PKC by inhibiting the movement of PKCα, PKCβ, and PKCζ in a concentration-dependent manner(Chao et al., 1998; Siomboing et al., 2001). As a subtype of the PKC family, PKCα affects tumor growth, the cell cycle, cell metastasis, and cell apoptosis (Leitges, 2007; Konopatskaya and Poole, 2010), which regulates cancer cells through the phosphorylation of P-gp (Zhang et al., 2003). CHE has been proven to be a highly effective and selective inhibitor of PKCα (Melling et al., 2009), inducing cell apoptosis by inhibiting PKCα, downregulating the transcription of MDR1, and reducing the expression of P-gp, which also reversed multidrug resistance (Cao et al., 2011). Furthermore, CHE, in addition to PKC inhibition, has a non-competitive inhibitory action on the human P2X7 receptor itself, which needs further research in the future (Shemon et al., 2004).
3.6 Inducing Autophagy-Mediated Cell Death
Autophagy is a form of programmed cell death (Tsujimoto and Shimizu, 2005) that involves many diseases and physiological processes, including cancer and ageing (Levine and Kroemer, 2008; Yang et al., 2011). Autophagy can be induced by ROS production, hypoxia, nutritional deficiencies, and viral infections (Azad et al., 2009). Therefore, inducing autophagy may be an essential strategy against cancer.
mTOR is a central regulator of autophagy and is related to cell proliferation and regulated by adenosine monophosphate-activated protein (AMP)–activated kinase (AMPK), which overactivates cancer cells. Medvetz et al. (2014) screened 6,700 compounds against mTOR and found that CHE had the most substantial killing effect on mTORC1-overactive cells, inducing autophagy by inhibiting mTORC1 and increasing AMPK (Kim et al., 2011), which forms autophagosomes and converts LC3I to LC3II (Tanida et al., 2008; Mah and Ryan, 2011; Si et al., 2020). Likewise, CHE has been shown to enhance the expression of LC3-II in NSCLC A549 and NCI-H1299 cells (Tang et al., 2017). In addition, ROS can trigger prodeath or prosurvival autophagy in cancer cells (Chen et al., 2008). CHE-induced ROS-mediated autophagy was found to contribute to apoptosis in NCI-H1299 cells, while CHE-induced autophagy through the production of ROS is a concomitant effect in A549 cells.
3.7 Chelerythrine as a Selective Telomeric G- Quadruplex DNA Stabilizer and Telomerase Inhibitor
DNA has attracted attention as a drug target or potential antitumor target. Some small molecules that can bind to DNA, such as cisplatin, have become clinically applied antitumor drugs. The pharmacological activity of CHE, especially its anticancer activity, is closely related to the structural characteristics of its molecules, which readily bind to the base pairs in the DNA structure. In vitro studies experimenting with CHE treatment on mouse spleen cells and lymphoid leukemia L1210 cells found that the drug has a damaging effect on DNA in a dose-dependent manner (Kaminskyy et al., 2008; Urbanová et al., 2009). Three DNA adducts were detected after incubation with CHE and calf thymus deoxyribonucleic acid (CT-DNA) in the presence of Sprague–Dawley rat liver microsomes and nicotinamide adenine dinucleotide phosphate (NADPH) (Stiborová et al., 2002). Subsequently, CHE was shown to interact with DNA, according to multiple experiments (Bai et al., 2008, Bai et al., 2006, Bai et al., 2014). CHE could bind with DNA [Ka=(1.04 ± 0.11)×106], where the particular binding site of CHE is a continuous GC pairing sequence (5′-TGGGGA-3′/3′-ACCCCT-5′), and CHE is reported to have a specific combination character with single nucleotide bulge DNA with C and T. CHE interacts with CT-DNA mainly through the interlayer, which shows its therapeutic prospects with the ability to insert the interlayer and stabilize the DNA double helix structure (Li et al., 2012).
Moreover, G-quadruplex has become one of the critical targets for developing antitumor drugs, and CHE binds selectively to the telomeric sequence G-quadruplex (Bai et al., 2014). DNA and RNA can fold into a variety of alternative conformations. A particular nucleic acid structure called G-quadruplex plays an important role in malignant transformation and cancer development (Kosiol et al., 2021). As a promising target for cancer therapy, molecular and cell biology have demonstrated the G-quadruplex formation with key biological processes ranging from genome instability and cancer (Varshney et al., 2020). CHE exerts antitumor activity by possessing a solid affinity for inhibiting the telomerase activity by substrate sequestration through G-quadruplex stabilization, which leads to fast proliferation of cells, thus promoting tumor cell death (Noureini et al., 2017). The binding selectivity of CHE toward G-quadruplexes is confirmed by both experimental and theoretical determination (Terenzi et al., 2019). In addition, CHE is reported to bind to non-telomeric G-quadruplex sequences also in the promoters of other genes, which binds to G-quadruplexes at promoters of VEGFA, BCL2, and KRAS genes and downregulates their expression (Jana et al., 2017). CHE binds to the ds poly (rA) by the mechanism of intercalation, not only interacting with DNA but also interacting with double-stranded RNA, which may also be one of the anticancer mechanisms (Pradhan et al., 2017).
3.8 Regulation of the MAPK Signaling Pathway
Mitogen-activated protein kinase (MAPK) pathways are viable targets that have been widely studied for cancer therapy. The extracellular signaling–regulated kinase (ERK) pathway is the most prominent and clinically utilized target, while the Jun N-terminal kinase (JNK) pathway and p38 pathway play critical modulatory roles in cancer cells. These pathways can regulate metabolism and epigenetics and change the response of cancer cells to both targeted therapies and chemotherapies (Lee et al., 2020).
CHE was reported to preferentially activate the JNK1 and P38 MAPK pathways in cervical cancer cells (HeLa), inducing cell apoptosis via the oxidative stress-related MEKK1, MKK4-dependent JNK1, and P38 pathways (Yu et al., 2000). CHE-induced cell apoptosis is mediated by activating the MKK4/JNK1 and MEKK1/P38 pathways in osteosarcoma cell lines. Likewise, CHE was further reported to activate the MEK/ERK1/2 pathway in a concentration–time-dependent manner, stimulating the ERK MAPK cascade to induce apoptosis, while P90RSK was found to be a downstream kinase of ERK1/2. The activation of ERK and cell apoptosis induced by CHE could be significantly reversed by MEK1-specific inhibitors (UO126 and PD98059) (Yang et al., 2008).
4 Role of Chelerythrine in a Variety of Human Cancers
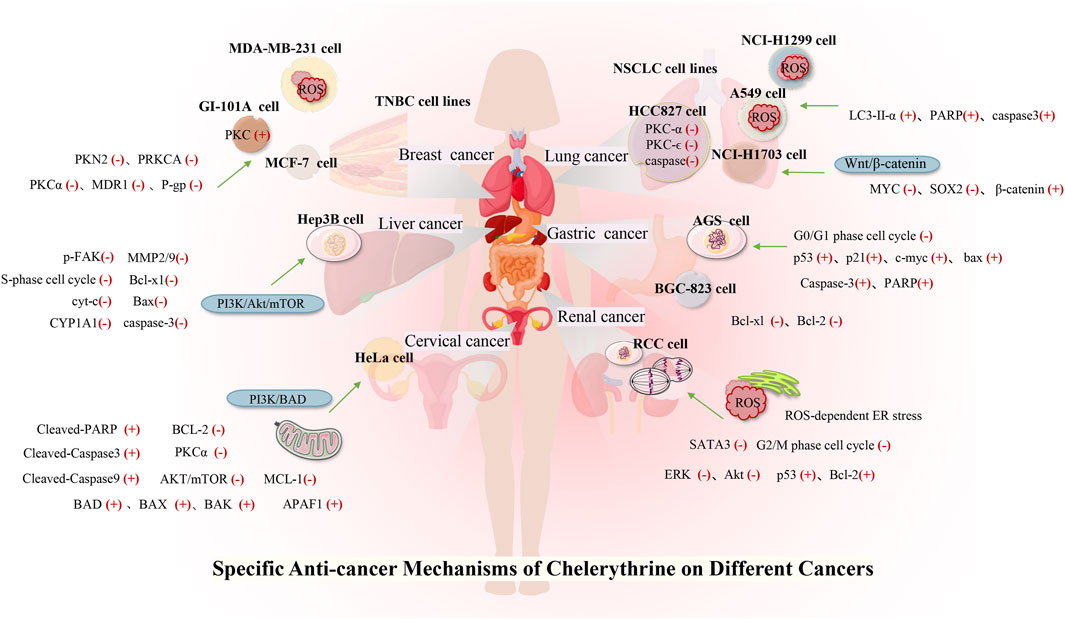
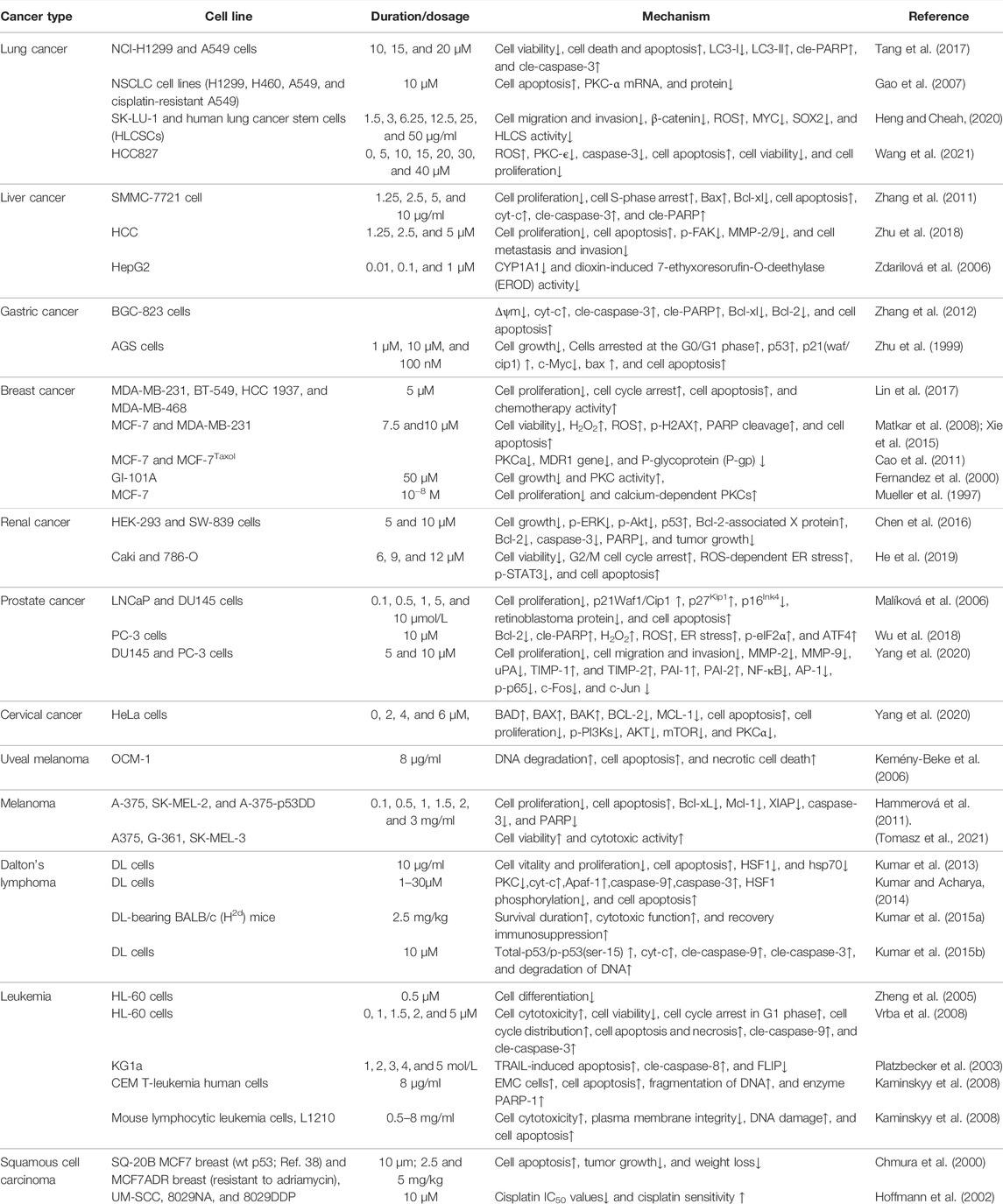
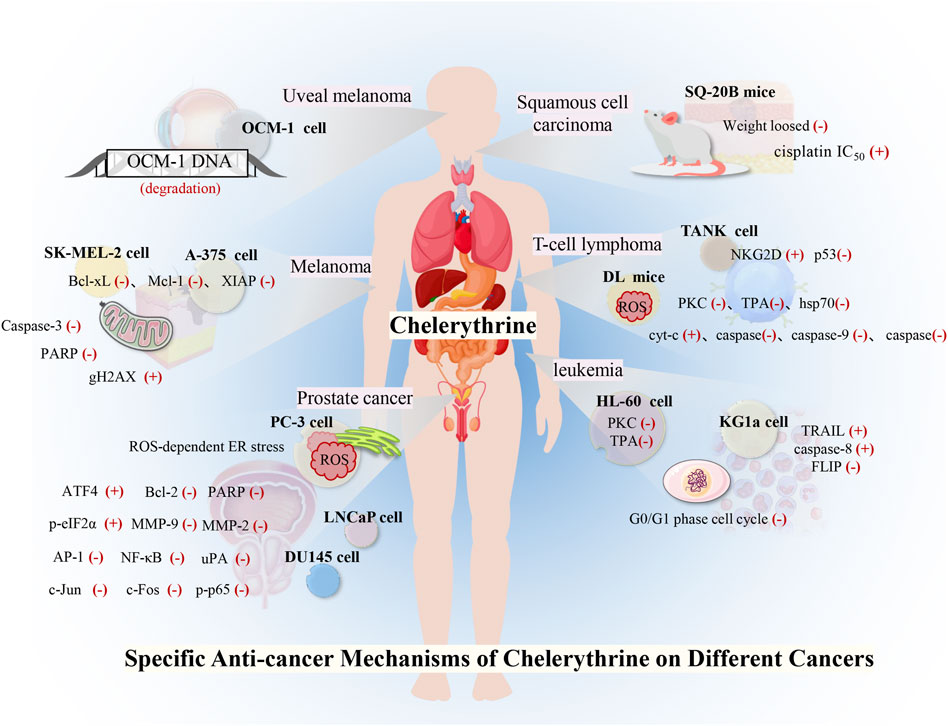
Many studies have investigated the role of CHE against different types of cancer. Drug discovery programs have identified CHE as the main antitumor drug candidate. CHE is a multitargeted agent with broad-spectrum antitumor effects that can be used as a wonder drug for treating cancers (Table 2). Some of the studies evaluating the antitumor potential of this drug are summarized as follows (Figures 4, 5).
4.1 Lung Cancer
Lung cancer is the most common malignant tumor, with 1.38 million deaths occurring each year. In lung cancers, approximately 80–85% are non–small cell lung cancer (NSCLC), and 10–15% are small cell lung cancer (Wan et al., 2018). Despite the continuous improvement of detection and therapeutic methods, NSCLC is still difficult to diagnose and has a poor prognosis. Reports stated that CHE efficiently enhanced the drug sensitivity of NSCLC cell lines (including cisplatin-resistant A549) to cisplatin by inhibiting the expression of PKC-α and elevating tumor cell apoptosis rates (Gao et al., 2007). CHE induced cell apoptosis in lung cancer cell lines (A549 and NCI-H1299) by suppressing the protein expression of LC3-II and increasing the protein expressions of PARP and cleaved caspase-3 (Tang et al., 2017). A549 cells have a higher antioxidant ability than NCI-H1299 cells (Sun et al., 2016), making it much easier to generate ROS in lung cell lines, which results in autophagic cell death (Martin and Barrett, 2002). CHE can affect ROS-mediated autophagy-induced cell death in lung cancer cell lines (Tang et al., 2017). Another study on the NSCLC cell line (HCC827) reported the efficacy of CHE in inducing apoptosis and inhibiting cell growth by inducing ROS release and downregulating caspase-3 and PKC-ϵ expression. CHE imparted anticancer effects on HCC827 by inhibiting PKC and caspase-3 activation, which were blocked by PMA, a PKC activator, thereby proving that CHE inhibited cancer cell viability and proliferation and induced cell apoptosis through the ROS/PKC-ϵ/caspase three pathway and glycolysis (Wang et al., 2021).
In addition, an in vivo study proved that CHE exerted specific anticancer effects on concentration adjustment to inhibit the Wnt/β-catenin pathway in different lung cancers and facilitate cancer cell apoptosis, inhibiting the proliferation and diminishing the self-renewal capacity of human lung cancer stem cells (HLCSCs) by downregulating β-catenin, SOX2, and MYC (Heng and Cheah, 2020; Wang et al., 2021). Owing to the inhibition of the tumorigenic activity of HLCSCs by CHE, which can inhibit cancer growth, the CHE concentration can be flexibly adjusted to protect against different specific targets in different cancers. A novel CHE analog, namely, compound 3f, was also found to induce dose-dependent G0/G1 cell cycle arrest, which enhanced selectivity against NSCLC tumor cells (Yang et al., 2016).
4.2 Liver Cancer
Most liver cancer patients have a definitive diagnosis and reach an advanced stage due to early unobvious symptoms. Currently, the small application range of targeted therapy drugs and recurrence and drug resistance after radiotherapy and ablation treatment lead to the unoptimistic overall treatment effect of advanced liver cancer, and the 5-year survival rate is low (Schlachterman et al., 2015).
CHE was reported to inhibit the proliferation of HCC cells and promote cell apoptosis, inhibiting metastasis and invasion of HCC in a dose-dependent manner by downregulating the expression of MMP-2/9 through the PI3K/Akt/mTOR pathway (Zhu et al., 2018). CHE not only significantly inhibits liver cancer cell proliferation through cell S-phase arrest, regulating the protein Bcl-2, increasing the proapoptotic protein Bax, and downregulating Bcl-xl but also disrupts the mitochondrial membrane potential. Activated caspase-3 and cleaved PARP result in apoptosis in a dose-dependent manner (Zhang et al., 2011). Moreover, some studies have pointed out that the biological effects of CHE may be related to the aryl hydrocarbon receptor (AhR). Dvořáka et al. (2006) found that CHE did not activate AhR in the rat hepatoma cell line H4IIE at any time or dose test and had no effect on the transcription of AhR. CHE exerted biological effects by inhibiting the catalytic activity of CYP1A1 in the human liver cancer cell line HepG2 but did not affect the expression of CYP1A1 (Zdarilová et al., 2006).
4.3 Gastric Cancer
Gastric cancer is the second leading cause of cancer-related deaths, owing to its high mortality (Lee et al., 2014). Gastric cancer has a higher level of PKC activity than normal tissues. As a PKC inhibitor, CHE can suppress cell growth and induce apoptosis in a gastric cancer cell line (AGS) by inducing G0/G1 phase arrest, as evaluated by the expressions of p53, p21 (waf/cip1), c-myc, and Bax (Zhu et al., 1999). The compound was also found to arrest the S phase, decrease Δψm, and release Cyt-c from mitochondria into the cytoplasm in a dose-dependent manner, which subsequently activated caspase-3 to cleave the PARP protein. CHE also imparted apoptotic effects on the human gastric cancer cell line BGC-823 by downregulating the expressions of Bcl-xl and Bcl-2 (Zhang et al., 2012).
4.4 Breast Cancer
Breast cancer has become the leading cause of female cancer death, and existing breast cancer chemotherapy drugs have limitations such as large adverse reactions, easy drug resistance, and lack of selectivity (DeSantis et al., 2019). Triple-negative breast cancer (TNBC) currently lacks therapy. One of the potential targets of TNBC is PKC, and PKN2 is highly expressed in TNBC. CHE showed potent anticancer activity against TNBC that overexpressed PKN2 and PRKCA by inhibiting cell growth and inducing apoptosis (Lin et al., 2017), selectively inhibiting growth with a half-inhibitory concentration (IC50) of 1.6 μM for MDA-MB-231 cells and increasing ROS generation, thereby inducing apoptosis (Matkar et al., 2008; Lin et al., 2017).
CHE (50 μM) can block the cell growth and activation of PKC induced by HCQ and prednisone treatment in GI-101A breast cancer cells (Fernandez et al., 2000). Selective stimulation of calcium-dependent PKCs has been reported to block the proliferation of MCF-7 cells (Mueller et al., 1997). CHE inhibited the PKC activity in MCF-7 and MCF-7Taxol cells, downregulated the level of MDR1, and reduced the level of P-gp (Cao et al., 2011). CHE, effectively extracting (UHPE) and isolating (PZRCCC), was more active against breast cancer cells than the positive control (vincristine = 5.04 μg/ml) (Ali et al., 2020).
4.5 Renal Cancer
Renal cancer accounts for 2%–4% of all adult malignant diseases worldwide, with a mortality rate of more than 40% (Znaor et al., 2015). Renal cell carcinoma (RCC) accounts for approximately 70% of renal cancer cases (Novick, 2007), and approximately half of patients are resistant to chemotherapy and radiotherapy (Znaor et al., 2015). CHE selectively killed human RCC cells via inhibition of cell viability and induction of G2/M phase arrest (Caki and 786-O), also showing proapoptotic properties in RCC through the activation of ROS-dependent ER stress and the inhibition of STAT3 phosphorylation (He et al., 2019). Another study on human renal cancer cell lines (SW-839) showed that CHE could inhibit the proliferation and growth of renal cancer cells in a time- and dose-dependent manner by decreasing the phosphorylation of ERK and Akt, upregulating p53 and Bcl-2, and cleaving caspase-3 and PARP protein. CHE effectively reduced tumor growth in a xenograft tumor model, perhaps indicating that CHE affects renal cancer via inhibition of the ERK activity (Chen et al., 2016).
4.6 Cervical Cancer
Cervical cancer is the most common female cancer worldwide. The potency of CHE against cervical cancer cells (HeLa) was evaluated by Yang et al. in 2020. They reported that CHE inhibited cell proliferation by triggering mitochondrial apoptosis through the PI3K/BAD signaling pathway, significantly increasing the activated expression of the BAD protein, upregulating the proapoptotic proteins BAX and BAK, and downregulating the antiapoptotic proteins BCL-2 and MCL-1 (Yang et al., 2020). Moreover, another study on HeLa cells reported the efficacy of CHE in inducing apoptosis by preventing the phosphorylation of PI3K and the suppression of AKT/mTOR and PKCα. CHE also triggered the mitochondrial “intrinsic” pathway by upregulating the expression of APAF1, cleaved-caspase3,9, and cleaved-PARP in HeLa cells (T. Yang et al., 2020).
4.7 Uveal Melanoma
Uveal melanoma, an intraocular malignant tumor that originates from melanocytes in the eyes, is an aggressive malignancy and easily metastasizes to other organs. CHE showed potent anticancer activity against uveal melanoma by catalyzing DNA fragmentation and apoptosis. CHE was reported to significantly induce DNA degradation and cell apoptosis of the human vein melanoma cell line OCM-1 and cause necrotic cell death in a dose-dependent manner (Kemény-Beke et al., 2006).
4.8 Head and Neck Squamous Cell Carcinoma
Head and neck squamous cell carcinoma (HNSCC) is a malignant tumor of squamous cell differentiation, and the incidence of HNSCC continues to rise (Ferlay et al., 2019; Johnson et al., 2020). Studies on HNSCC cell lines showed that CHE induced rapid radiation and chemotherapy-resistant cell apoptosis in vitro, while CHE significantly inhibited tumor growth in HNSCC tumor model mice with xenotransplantation, with a weight loss of less than 10% (Chmura et al., 2000). CHE interacted with PKC and significantly decreased cisplatin IC50 values in six cell lines derived from HNSCC in combination with cisplatin (IC50 = 0.4–5.8 μg/ml), which also increased the cisplatin sensitivity of the HNSCC cell lines (Hoffmann et al., 2002).
4.9 Melanoma
Although comprising only approximately 1% of skin cancers, melanoma is the most dangerous skin cancer with a high mortality rate, and the incidence has increased steadily (Dzwierzynski, 2013). Sanguinaria canadensis has been recorded to treat melanoma, and the main component, benzophenanthridine alkaloids, is a drug that can inhibit the growth and viability of melanoma cells through a variety of molecular mechanisms. Among these mechanisms, the anticancer potential of CHE was evaluated in human melanoma A-375 and SK-MEL-2 cell lines, where the drug was found to facilitate cell apoptosis by suppressing the expressions of antiapoptotic proteins (Bcl-xL, Mcl-1, and XIAP), decreasing mitochondrial membrane potential, and cleaving caspase-3 and PARP. The phosphorylation of histone H2AX was detected to evaluate the DNA damage related to behavioral activation therapy (BA) treatment, and CHE was further reported to increase the expression of gH2AX and regulate caspase-3 cleavage at different levels (Hammerová et al., 2011). CHE showed strong cytotoxic activity against human melanoma cell lines (A375, G-361, and SK-MEL-3) with IC50 values below 0.55 μg/ml compared with anticancer drugs (etoposide, cisplatin, and hydroxyurea), indicating that CHE has high anticancer potential in melanoma (Tuzimski et al., 2021).
4.10 T-Cell Lymphoma
T-cell lymphoma is characterized by highly deleterious, invasive, and rapid growth due to its unique etiology. CHE was reported not only to reduce the vitality of murine T-cell lymphoma (DL) cells but also to induce cell apoptosis. CHE also blocked the PKC activation that inhibited heat shock factor 1 (HSF1) and hsp70 expression (Kumar et al., 2013). Another study demonstrated that CHE induced cell apoptosis and increased ROS generation by inducing the permeability and rupture of the mitochondrial membrane, thereby inducing apoptosis of DL cells by releasing cyt-c and activating Apaf-1 and the cleavage of caspase-9,3 (Kumar and Acharya, 2014).
In addition, CHE significantly increased the protein and mRNA expressions of total p53/phospho-p53, which led to the induction of apoptosis by the p53 pathway, which was further confirmed by the p53 knockdown of DL cells (Kumar et al., 2015b). In vitro, CHE was also found to significantly delay the progressive growth of DL and increase the survival period of DL-bearing BALB/c (H2d) mice. The in vivo administration of CHE (2.5 mg/kg) results in increased cytotoxic function of TANK cells and recovery of immunosuppression caused by tumor invasion (Kumar et al., 2015a).
4.11 Leukemia
Leukemia is a type of malignant tumor affecting the hematopoietic system. PKC is involved in the pharmacological activity of 12-O-tetradecanoylphorbol-13-acetate (TPA)–induced growth inhibition and apoptosis in myeloid leukemia. As a broad inhibitor of the PKC family, CHE significantly decreased cell differentiation in the human promyelocytic leukemia (HL-60) cell line, which is induced by the combination of TPA and capsaicin (Zheng et al., 2005). A study on HL-60 cells stated that CHE exhibited stronger antiproliferative activity than other alkaloids (Hatae et al., 2015). CHE was reported to have a cytotoxic effect on HL-60 cells, which results in decreased cell viability in a dose-dependent manner and accumulation of the cell cycle at the G1 phase. CHE also induced apoptosis of HL-60 cells by activating mitochondrial apoptotic pathways (Vrba et al., 2008).
Recent data have shown that CHE promotes the FAS-mediated cell state in the immature (CD34+/38-) myeloid cell line KG1a. CHE induced KG1a cells to be sensitive to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), which activated caspase-8 and downregulated FLIP long and short (Platzbecker et al., 2003). Another study demonstrated the typical characteristics of apoptosis in CEM T-leukemia human cells treated with CHE through the changes in the microstructure of EMC cells, which led to the further development of apoptosis, including fragmentation of DNA and reparation enzyme PARP-1 (Kaminskyy et al., 2008). CHE was also reported to induce DNA damage, cell cytotoxicity, and disruption of plasma membrane integrity in L1210 leukemic cells, which were more sensitive to the toxic effect of CHE than normal mouse spleen cells. These results suggested that CHE possesses slightly selective toxicity against cancer cells without affecting normal cells (Kaminskyy et al., 2008).
4.12 Prostate Cancer
Prostate cancer is the most common malignancy in men worldwide (Prashanth, 2019). Advanced localized, recurrent, and metastatic diseases often portend poor outcomes (La et al., 2019). The endoplasmic reticulum (ER) plays an important role in the apoptosis signaling pathway (Ryu et al., 2017), and ROS levels are related to the ER stress pathway. CHE treatment was found to exhibit excellent anticancer effects in prostate cancer cells by inducing ROS-dependent ER stress (Wu et al., 2018), which reduced Bcl-2 and increased cleaved PARP. ROS accumulation in PC-3 prostate cancer cells via increased intracellular H2O2 levels was achieved by CHE. In addition, CHE induced ER stress by increasing the levels of p-eIF2α and ATF4 in a time-dependent manner (Ryu et al., 2017).
CHE was reported to inhibit cell growth and regulate the expression of cell cycle- and apoptosis-related proteins in human prostate cancer cell lines (LNCaP and DU145) (Malíková et al., 2006). CHE was further found to have a significant effect on inhibiting the invasion and metastasis of these androgen-independent cancer cells at concentrations of 5–10 μM. The compound decreased the MMP-9, MMP-2, and uPA proteins and augmented the expressions of PAI-1 and PAI-2. Meanwhile, CHE also suppressed NF-κB and AP-1, which was demonstrated by the decreased expressions of p-p65, c-Jun protein expression, and c-Fos (Yang et al., 2020).
5 Synergistic Interactions of Chelerythrine With Other Agents
Although the anticancer activity of CHE has been confirmed, CHE has not been widely used in human clinical trials. Therefore, researchers need to look for new CHE values from other perspectives. Many natural products have synergistic effects with other listed anticancer drugs (Li et al., 2017). The main advantage of combined therapy is reducing the dose and toxicity of chemotherapy while maintaining or even increasing its efficacy. Several studies have explored the advantages of combining natural compounds with classical chemotherapeutic drugs (Cai et al., 2002; Efferth et al., 2002).
CHE was found to enhance the inhibition of cell proliferation of the chemotherapy agent taxol on TNBC cells. Compared with the single-drug treatment, the combination treatment of dual drugs significantly reduced cell proliferation, indicating a promising therapeutic schedule for TNBC. The combination treatment of CHE with taxol had a synergistic effect with combination index (CI) values at ED50 (0.75190–1.13763) (Lin et al., 2017).
Combination treatment of CHE with cisplatin significantly decreased cisplatin IC50 values and increased cisplatin sensitivity in HNSCC cell lines, and the IC50 value of CHE was between 8.5 μM and 13.6 μM. CHE (IC50 = 10 μM) combined with increasing doses of cisplatin demonstrated an additive effect compared with the theoretical additive dose-response curves. However, the single use of CHE was always more effective in inducing cancer cell apoptosis than the combined treatment with cisplatin (Hoffmann et al., 2002).
The potentiated inhibitory effect of CHE combined with erlotinib was also evident against NSCLC cell lines, which resulted in a significant decrease in malignant cell features. The combination treatment effectively delayed tumor growth and blocked EGFR by decreasing the phosphorylation of STAT3, p38 MAPK, ERK1/2, and BAD proteins, while the IC50 value of CHE was between HCC827 (IC50=5.0 ± 0.48 μM), SK-MES-1 (IC50=6.35 ± 1.26 μM), and A459 (IC50=7.78 ± 0.56 μM) (He et al., 2017). In SK-MES-1 and A459 cells, which are less sensitive to erlotinib, CHE showed potentiated inhibitory effects compared with erlotinib. Similarly, growth inhibition and apoptosis induction in tumor cells showed significant synergistic antitumor effects in NSCLC cells when CHE was combined with cisplatin. CHE (72.4%) also had a better effect on inhibiting the growth of tumors than the single use of cisplatin (64.3%) (Gao et al., 2007).
These studies provide a new perspective on the anticancer therapeutic strategy of CHE in combination therapy. At present, studies on the synergy of CHE with other agents are limited to the abovementioned limited cancers. For CHE as a broad-spectrum anticancer drug, more options can be explored in the future.
6 Toxicity Study and Clinical Application of Chelerythrine
6.1 Toxicity and Security of Chelerythrine
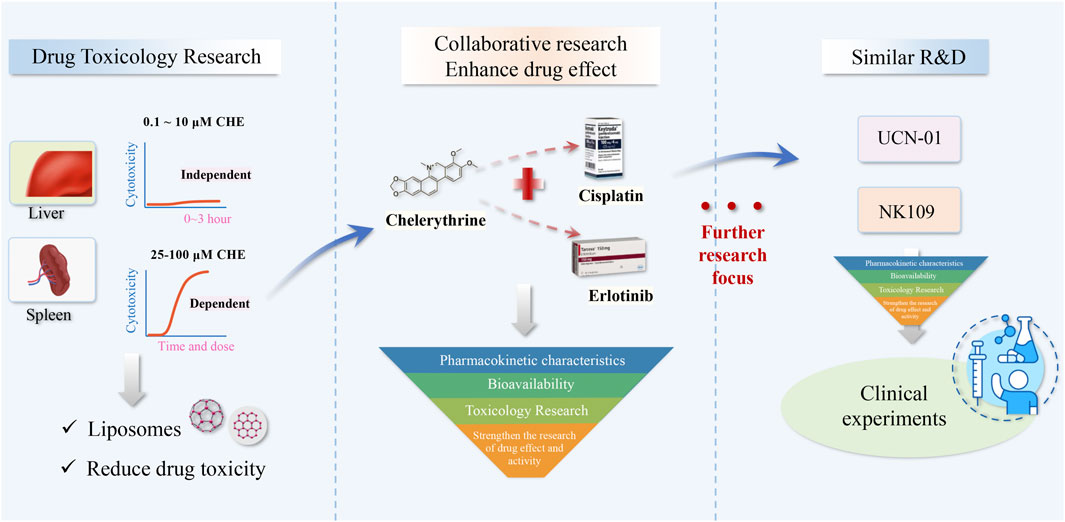
CHE has abundant pharmacological activity in the treatment of cancers but has been greatly restricted in clinical use because of its toxic side effects and other shortcomings. Despite extensive investigations on the role and use of CHE in the prevention and treatment of cancers, only a few studies have mentioned its toxicity.
We also collected the toxicity reports of CHE from the literature, which provided useful data for further research and development of new drugs containing CHE. In the works of Huang et al., high-performance liquid chromatography–tandem mass spectrometry (HPLC–MS/MS) was used to detect the distribution of CHE in vivo and demonstrated that the concentrations of CHE in the liver after 48 h and spleen after 24 h were 20.64 ng/ml and 44.04 ng/ml, respectively, higher than those of other tissues (<15ng/g). The results also indicated that the metabolic enzymes of XO, NQO1, NQO2, and carbonyl reductase are involved in CHE reduction (Huang et al., 2021). Another study demonstrated that CHE triggered acute hepatotoxicity at a dose of 10 mg/kg/day, and any liver damage was not observed with the administration of 0.2 mg/kg intraperitoneally (i.p.) over 56 days (Ulrichová et al., 1996). Furthermore, they also demonstrated the nontoxic nature of CHE in human vs. porcine liver cells at dosages of approximately 0.1 and 10 μM in 0–3 h of incubation, while CHE showed dose–time-dependent toxicity within the range of 25–100 μM and mitochondrial dehydrogenase activity and the cellular glutathione levels suggesting that mitochondria are unlikely to be a primary target for CHE in the cell (Ulrichová et al., 2001). In addition, the destruction of cellular calcium homeostasis by CHE was observed with a notable Ca2+-ATPase (SERCA1)-inhibitory activity, suggesting a possible cause of the CHE cytotoxicity (Vieira et al., 2015).
Another experiment investigated the most toxic Chelidonium majus alkaloids (CALs) in rat hepatocytes and found that CHE (EC20 ≤ 2 μM) was one of the most toxic compounds, indicating a marked structure–toxicity relationship in hepatocyte cells (Gao et al., 2019). Studies have also indicated that CHE was safe under an average daily oral dose of 5 mg/kg, which did not cause any genotoxicity or hepatotoxicity (Kosina et al., 2003). However, different doses of CHE were administered to experimental Wistar rats, and the results showed that CHE increased cumulative mortality and had long-term toxicity on lung tissues in a dose-dependent manner. CHE can aggravate toxic lung damage at high dosages (>=5.6 mg/kg), possibly via NF-κB activation and the production of inflammatory cytokines (Liu et al., 2017). These studies have revealed that the toxicity of CHE may be related to the dosage and emphasized that a thorough investigation of long-term medication is needed.
Hence, controlling and reducing drug toxicity is the key aspect for CHE to enter human clinical research. Current toxicological studies provide us with data on safe doses of CHE. Recent studies provide some new materials that can reduce the toxicity of CHE. Pharmacokinetic studies have revealed that CHE long circulation liposomes (CHELPs) modified with polyethylene glycol remained stable and exhibited sustained drug release while increasing the bioavailability and alleviating the toxicity of CHE, which showed a good therapeutic effect against cancer (Li et al., 2013; Wang et al., 2021). This study showed that nanostructured material may be an effective method to reduce chelerythrine toxicity and may contribute to the development of novel drugs in clinical trials.
6.2 Pharmacokinetics of Chelerythrine
In recent years, there have been many studies on the pharmacokinetics of CHE in animals, but there is still a lack of human research. Some studies were conducted to investigate the single- and multiple-dose pharmacokinetics of chelerythrine (CHE) and its metabolite in animals. The AUC value for CHE was 0.52 ± 0.09 (ng/ml hr), and the calibration curves were linear over the concentration range of 0.5–100.0 ng/g for CHE in broiler chickens (Xie et al., 2015; Hu et al., 2019). After oral and IM administrations in pigs, CHE rapidly reached Cmax of 5.04 ± 1.00 ng/ml at 1.83 ± 0.26 h and Cmax of 69.79 ± 15.41 ng/ml at 0.42 ± 0.13 h, respectively. The half-life (T1/2) was 2.03 ± 0.26 h for CHE (Zhao et al., 2021). The results of these studies revealed that CHE is metabolized rapidly. At the same time, pharmacokinetic studies were also carried out from the extracts of these traditional plants, such as Toddalia asiatica (Qiu et al., 2022), Chelidonium majus L.(Zhou et al., 2013), and Macleaya cordata extracts (Shen et al., 2022). In pharmacokinetic studies of CHE in rat plasma through UPLC-Q-TOF-MS and UPLC-MS/MS after the oral administration, the results showed the plasma drug concentration of CHE was very low (Lin et al., 2020). Recent studies proved that the biomaterial might be a suitable method to enhance the bioavailability and improve the therapeutic efficacy of CHE in vivo. The relative bioavailability of CHE in liposomes (CHELPs) was significantly increased, and CHELP has a lower clearance rate and higher absorption concentration than the free drug (Li et al., 2013b; Wang et al., 2021).
6.3 Clinical Application
In fact, clinical evidence remains scarce, which is typical for many natural products isolated from medicinal plants, and CHE is no exception. At present, UCN-01 and NK109, CHE analogs, have entered the clinical trial stage as antitumor drugs (Gojo et al., 2013; Ma et al., 2013), and most of these drugs are used in combination with classical chemotherapeutic drugs. Among them, many pieces of evidence of efficacy suggest that UCN-01 has a notable curative effect in combination with classical chemotherapeutic drugs in patients with solid tumors (Sausville et al., 2001; Kortmansky et al., 2005; Hotte et al., 2006). Nonetheless, CHE is effective against a vast range of cancers and is expected to be used as a preparation for the clinical treatment of cancer, which needs further development based on pharmacological activity research. Further optimization of its structure and development of related analogs are also feasible methods. Studies related to the pharmacokinetic properties, toxicity, and bioavailability of CHE are of utmost importance and can provide a scientific theoretical basis for its clinical development and utilization, which has certain practical significance.
The toxicity studies, combination therapy, and clinical application of CHE indicate the focus of future research.
7 Conclusion
In conclusion, similar to artemisinin and paclitaxel, the experience of traditional Chinese medicine and herbs has provided us with a large number of abundant effective compounds, and further research and optimized composition will contribute to human health. Extensive efforts have been devoted to identifying the bioactivity and mechanism of CHE and publishing its effectiveness in the treatment of different cancers. CHE was reported to act upon numerous molecular targets and mechanisms that are the key roles associated with pathogenesis in cancers. Therefore, CHE has shown immense potential in the treatment of cancers. The antitumor activity of CHE is the comprehensive effect of proliferation, invasiveness, and apoptosis of tumor cells, which inhibits tumor growth through multiple molecular pathways with few side effects due to its proapoptotic potential and makes CHE a suitable candidate for the treatment of cancers.
CHE has shown immense potential in the treatment of cancers; however, CHE has not been widely used in human clinical trials due to its shortcomings, such as drug toxicity. Therefore, researchers need to look for new values from other perspectives. In addition, as an underestimated anticancer drug, more studies should broaden the scope of traditional uses and provide better preparations, particularly binding to different categories of active molecules, which requires more extensive investigation and interdisciplinary efforts. At present, studies on the synergy of CHE with other agents identified that combined therapy is reducing the dose and toxicity while maintaining or even increasing its efficacy, and nanostructured material may be an effective method to reduce CHE toxicity and increase the bioavailability. Further in-depth pharmacokinetic studies on humans and toxicology research, the mechanism of combination therapy, and research on new CHE-based biomaterials for reducing drug toxicity and structurally optimized analogs are required to enhance the efficacy and safety of CHE, and more clinical studies are required in the future to validate preclinical studies of CHE as an antitumor strategy.
This review summarized the anticancer abilities and the antitumor mechanism of CHE. It also highlighted the limitations and the current problems, and we believe that this review can provide support for the clinical application of a new anticancer drug in the future.
Author Contributions
JT and JZ conceived the study. NC, YQ, XM, and XX contributed to the design and execution. QL, TX, and JX helped with the execution of this manuscript. All authors read and approved the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (Nos. 82074397, 81804066, and 82174346), the Xinglin Scholar Research Promotion Project of Chengdu University of TCM (No. QNXZ2019017), the Hundred Talents Program of the Hospital of Chengdu University of Traditional Chinese Medicine (No. 21-Y17 and 20-L01), and the Project of Sichuan Administration of Traditional Chinese Medicine (No. 2021MS104).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AMPK, adenosine monophosphate-activated protein-activated kinase; CHE, chelerythrine; ER, endoplasmic reticulum; ERK, extracellular signaling-regulated kinase; HNSCC, head and neck squamous cell carcinoma; IC50, a concentration that causes 50% inhibition; JNK, Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MMP, mitochondrial membrane potential; NSCLC, non–small cell lung cancer; PARP, poly-ADP-ribose polymerase; PKC, protein kinase C; ROS, reactive oxygen species; TNBC, triple-negative breast cancer; RCC, renal cell carcinoma.
References
Achkar, I. W., Mraiche, F., Mohammad, R. M., and Uddin, S. (2017). Anticancer Potential of Sanguinarine for Various Human Malignancies. Future Med. Chem. 9, 933–950. doi:10.4155/fmc-2017-0041
Ali, I., Li, J., Cui, L., Zhao, H., He, Q., and Wang, D. (2020). Efficient Extraction and Purification of Benzo[c]phenanthridine Alkaloids from Macleaya Cordata (Willd) R. Br. By Combination of Ultrahigh Pressure Extraction and pH-Zone-Refining Counter-current Chromatography with Anti-breast Cancer Activity In Vitro. Phytochem. Anal. 32, 423–432. doi:10.1002/pca.2990
Azad, M. B., Chen, Y., and Gibson, S. B. (2009). Regulation of Autophagy by Reactive Oxygen Species (ROS): Implications for Cancer Progression and Treatment. Antioxid. Redox Signal 11, 777–790. doi:10.1089/ars.2008.2270
Bai, L. P., Cai, Z., Zhao, Z. Z., Nakatani, K., and Jiang, Z. H. (2008). Site-specific Binding of Chelerythrine and Sanguinarine to Single Pyrimidine Bulges in Hairpin DNA. Anal. Bioanal. Chem. 392, 709–716. doi:10.1007/s00216-008-2302-7
Bai, L. P., Hagihara, M., Nakatani, K., and Jiang, Z. H. (2014). Recognition of Chelerythrine to Human Telomeric DNA and RNA G-Quadruplexes. Sci. Rep. 4, 6767. doi:10.1038/srep06767
Bai, L. P., Zhao, Z. Z., Cai, Z., and Jiang, Z. H. (2006). DNA-binding Affinities and Sequence Selectivity of Quaternary Benzophenanthridine Alkaloids Sanguinarine, Chelerythrine, and Nitidine. Bioorg Med. Chem. 14, 5439–5445. doi:10.1016/j.bmc.2006.05.012
Bambagiotti-Alberti, M., Pinzauti, S., Moneti, G., Gratteri, P., Coran, S. A., and Vincieri, F. F. (1991). Characterization of Sanguinaria Canadensis L. Fluid Extract by FAB Mass Spectrometry. J. Pharm. Biomed. Anal. 9, 1083–1087. doi:10.1016/0731-7085(91)80048-e
Banik, K., Ranaware, A. M., Deshpande, V., Nalawade, S. P., Padmavathi, G., Bordoloi, D., et al. (2019). Honokiol for Cancer Therapeutics: A Traditional Medicine that Can Modulate Multiple Oncogenic Targets. Pharmacol. Res. 144, 192–209. doi:10.1016/j.phrs.2019.04.004
Basu, P., Bhowmik, D., and Suresh Kumar, G. (2013). The Benzophenanthridine Alkaloid Chelerythrine Binds to DNA by Intercalation: Photophysical Aspects and Thermodynamic Results of Iminium versus Alkanolamine Interaction. J. Photochem Photobiol. B 129, 57–68. doi:10.1016/j.jphotobiol.2013.09.011
Bhuiya, S., Pradhan, A. B., Haque, L., and Das, S. (2016). Molecular Aspects of the Interaction of Iminium and Alkanolamine Forms of the Anticancer Alkaloid Chelerythrine with Plasma Protein Bovine Serum Albumin. J. Phys. Chem. B 120, 5–17. doi:10.1021/acs.jpcb.5b07818
Bishayee, A., and Sethi, G. (2016). Bioactive Natural Products in Cancer Prevention and Therapy: Progress and Promise. Semin. Cancer Biol. 40-41, 1–3. doi:10.1016/j.semcancer.2016.08.006
Biswas, S. J., Bhattacharjee, N., and Khuda-Bukhsh, A. R. (2008). Efficacy of a Plant Extract (Chelidonium Majus L.) in Combating Induced Hepatocarcinogenesis in Mice. Food Chem. Toxicol. 46, 1474–1487. doi:10.1016/j.fct.2007.12.009
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 68, 394–424. doi:10.3322/caac.21492
Cai, H. B., Dai, F. G., Min, Q. F., Shi, M., Miao, J. X., and Luo, R. C. (2002). Clinical Study of the Effects of Radiotherapy in Combination with Traditional Chinese Medicine on Non-small Cell Lung Cancer. Di Yi Jun Yi Da Xue Xue Bao 22, 1112–1113.
Cairns, R. A., Harris, I. S., and Mak, T. W. (2011). Regulation of Cancer Cell Metabolism. Nat. Rev. Cancer 11, 85–95. doi:10.1038/nrc2981
Cao, Z., Wang, L. J., Wu, M. H., Jiao, Y., Sun, Y. J., and Guo, J. (2011). Mechanism Governing Reversal of Multidrug Resistance in Human Breast Carcinoma Cells by Chelerythrine. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 33, 45–50.
Capistrano I, R., Wouters, A., Lardon, F., Gravekamp, C., Apers, S., and Pieters, L. (2015). In Vitro and In Vivo Investigations on the Antitumour Activity of Chelidonium Majus. Phytomedicine 22, 1279–1287. doi:10.1016/j.phymed.2015.10.013
Chan, S. L., Lee, M. C., Tan, K. O., Yang, L. K., Lee, A. S., Flotow, H., et al. (2003). Identification of Chelerythrine as an Inhibitor of BclXL Function. J. Biol. Chem. 278, 20453–20456. doi:10.1074/jbc.C300138200
Chao, M. D., Chen, I. S., and Cheng, J. T. (1998). Inhibition of Protein Kinase C Translocation from Cytosol to Membrane by Chelerythrine. Planta Med. 64, 662–663. doi:10.1055/s-2006-957545
Chen, W., Zhang, R., Lei, S., Zuo, H., Chen, Shi. Ji., and Chang, Y. (2020). Advanced in Chemical Constituents and Bioactivities of Macleaya Cordata. Chin. J. Exp. Traditional Med. Formulae 26, 243–250.
Chen, X. M., Zhang, M., Fan, P. L., Qin, Y. H., and Zhao, H. W. (2016). Chelerythrine Chloride Induces Apoptosis in Renal Cancer HEK-293 and SW-839 Cell Lines. Oncol. Lett. 11, 3917–3924. doi:10.3892/ol.2016.4520
Chen, Y., McMillan-Ward, E., Kong, J., Israels, S. J., and Gibson, S. B. (2008). Oxidative Stress Induces Autophagic Cell Death Independent of Apoptosis in Transformed and Cancer Cells. Cell. Death Differ. 15, 171–182. doi:10.1038/sj.cdd.4402233
Chen, Y. Z., Liu, G. Z., Shen, Y., Chen, B., and Zeng, J. G. (2009). Analysis of Alkaloids in Macleaya Cordata (Willd.) R. Br. Using High-Performance Liquid Chromatography with Diode Array Detection and Electrospray Ionization Mass Spectrometry. J. Chromatogr. A 1216, 2104–2110. doi:10.1016/j.chroma.2008.08.066
Chmura, S. J., Dolan, M. E., Cha, A., Mauceri, H. J., Kufe, D. W., and Weichselbaum, R. R. (2000). In Vitro and In Vivo Activity of Protein Kinase C Inhibitor Chelerythrine Chloride Induces Tumor Cell Toxicity and Growth Delay In Vivo. Clin. Cancer Res. 6, 737–742.
Chmura, S. J., Mauceri, H. J., Advani, S., Heimann, R., Beckett, M. A., Nodzenski, E., et al. (1997). Decreasing the Apoptotic Threshold of Tumor Cells through Protein Kinase C Inhibition and Sphingomyelinase Activation Increases Tumor Killing by Ionizing Radiation. Cancer Res. 57, 4340–4347.
Colombo, M. L., and Bosisio, E. (1996). Pharmacological Activities of Chelidonium Majus L. (Papaveraceae). Pharmacol. Res. 33, 127–134. doi:10.1006/phrs.1996.0019
Covarrubias, L., Hernández-García, D., Schnabel, D., Salas-Vidal, E., and Castro-Obregón, S. (2008). Function of Reactive Oxygen Species during Animal Development: Passive or Active? Dev. Biol. 320, 1–11. doi:10.1016/j.ydbio.2008.04.041
Croaker, A., King, G. J., Pyne, J. H., Anoopkumar-Dukie, S., and Liu, L. (2016). Sanguinaria Canadensis: Traditional Medicine, Phytochemical Composition, Biological Activities and Current Uses. Int. J. Mol. Sci. 17, 1414. doi:10.3390/ijms17091414
Deljanin, M., Nikolic, M., Baskic, D., Todorovic, D., Djurdjevic, P., Zaric, M., et al. (2016). Chelidonium Majus Crude Extract Inhibits Migration and Induces Cell Cycle Arrest and Apoptosis in Tumor Cell Lines. J. Ethnopharmacol. 190, 362–371. doi:10.1016/j.jep.2016.06.056
Deng, G., and Cassileth, B. (2013). Complementary or Alternative Medicine in Cancer Care-Myths and Realities. Nat. Rev. Clin. Oncol. 10, 656–664. doi:10.1038/nrclinonc.2013.125
DeSantis, C. E., Ma, J., Gaudet, M. M., Newman, L. A., Miller, K. D., Goding Sauer, A., et al. (2019). Breast Cancer Statistics, 2019. CA Cancer J. Clin. 69, 438–451. doi:10.3322/caac.21583
Duraipandiyan, V., and Ignacimuthu, S. (2009). Antibacterial and Antifungal Activity of Flindersine Isolated from the Traditional Medicinal Plant, Toddalia Asiatica (L.) Lam. J. Ethnopharmacol. 123, 494–498. doi:10.1016/j.jep.2009.02.020
Dvořáka, Z., Sovadinováb, I., Bláhab, L., Giesyc, J. P., and Ulrichová, J. (2006). Quaternary Benzo[c]phenathridine Alkaloids Sanguinarine and Chelerythrine Do Not Affect Transcriptional Activity of Aryl Hydrocarbon Receptor: Analyses in Rat Hepatoma Cell Line H4IIE.Luc. Food Chem. Toxicol. 44, 1466–1473.
Dzwierzynski, W. W. (2013). Managing Malignant Melanoma. Plast. Reconstr. Surg. 132, 446e–460e. doi:10.1097/PRS.0b013e31829ad411
Efferth, T., Davey, M., Olbrich, A., Rücker, G., Gebhart, E., and Davey, R. (2002). Activity of Drugs from Traditional Chinese Medicine toward Sensitive and MDR1- or MRP1-Overexpressing Multidrug-Resistant Human CCRF-CEM Leukemia Cells. Blood Cells Mol. Dis. 28, 160–168. doi:10.1006/bcmd.2002.0492
Ernst, E., and Schmidt, K. (2005). Ukrain - a New Cancer Cure? A Systematic Review of Randomised Clinical Trials. BMC Cancer 5, 69. doi:10.1186/1471-2407-5-69
Ferlay, J., Colombet, M., Soerjomataram, I., Mathers, C., Parkin, D. M., Piñeros, M., et al. (2019). Estimating the Global Cancer Incidence and Mortality in 2018: GLOBOCAN Sources and Methods. Int. J. Cancer 144, 1941–1953. doi:10.1002/ijc.31937
Fernandez, Y., Ramakrishnan, R., and Rathinavelu, A. (2000). Effect of Systemic Lupus Erythematosus (SLE) Treatment Drugs on GI-101A Breast Tumor Cell Growth. Life Sci. 67, 567–575. doi:10.1016/s0024-3205(00)00644-5
Gansauge, F., Ramadani, M., Pressmar, J., Gansauge, S., Muehling, B., Stecker, K., et al. (2002). NSC-631570 (Ukrain) in the Palliative Treatment of Pancreatic Cancer. Results of a Phase II Trial. Langenbecks Arch. Surg. 386, 570–574. doi:10.1007/s00423-001-0267-5
Gao, L., Schmitz, H. J., Merz, K. H., and Schrenk, D. (2019). Characterization of the Cytotoxicity of Selected Chelidonium Alkaloids in Rat Hepatocytes. Toxicol. Lett. 311, 91–97. doi:10.1016/j.toxlet.2019.04.031
Gao, Z., Tang, L., Su, B., Sha, H., and Han, B. (2007). Effects of Protein Kinase C Inhibitor,chelerythrine Chloride,on Drug-Sensitivity of NSCLC Cell Lines. Zhongguo Fei Ai Za Zhi 10, 455–460. doi:10.3779/j.issn.1009-3419.2007.06.02
Gao, Z. Q., Han, B. H., Sha, H. F., Shi, Z. Y., Yang, X. H., and Feng, J. X. (2010). Effects of a Protein Kinase C Inhibitor Combined with Cisplatin on Non-small Cell Lung Cancer. Zhonghua Jie He He Hu Xi Za Zhi 33, 284–288.
Gilca, M., Gaman, L., Panait, E., Stoian, I., and Atanasiu, V. (2010). Chelidonium Majus-Aan Integrative Review: Traditional Knowledge versus Modern Findings. Forsch Komplementmed 17, 241–248. doi:10.1159/000321397
Gojo, I., Perl, A., Luger, S., Baer, M. R., Norsworthy, K. J., Bauer, K. S., et al. (2013). Phase I Study of UCN-01 and Perifosine in Patients with Relapsed and Refractory Acute Leukemias and High-Risk Myelodysplastic Syndrome. Investig. New Drugs 31, 1217–1227. doi:10.1007/s10637-013-9937-8
Habermehl, D., Kammerer, B., Handrick, R., Eldh, T., Gruber, C., Cordes, N., et al. (2006). Proapoptotic Activity of Ukrain Is Based on Chelidonium Majus L. Alkaloids and Mediated via a Mitochondrial Death Pathway. BMC Cancer 6, 14. doi:10.1186/1471-2407-6-14
Hammerová, J., Uldrijan, S., Táborská, E., and Slaninová, I. (2011). Benzo[c]phenanthridine Alkaloids Exhibit Strong Anti-proliferative Activity in Malignant Melanoma Cells Regardless of Their P53 Status. J. Dermatological Sci. 62, 22–35.
Hatae, N., Fujita, E., Shigenobu, S., Shimoyama, S., Ishihara, Y., Kurata, Y., et al. (2015). Antiproliferative Activity of O4-Benzo[c]phenanthridine Alkaloids against HCT-116 and HL-60 Tumor Cells. Bioorg Med. Chem. Lett. 25, 2749–2752. doi:10.1016/j.bmcl.2015.05.031
Havelek, R., Seifrtova, M., Kralovec, K., Krocova, E., Tejkalova, V., Novotny, I., et al. (2016). Comparative Cytotoxicity of Chelidonine and Homochelidonine, the Dimethoxy Analogues Isolated from Chelidonium Majus L. (Papaveraceae), against Human Leukemic and Lung Carcinoma Cells. Phytomedicine 23, 253–266. doi:10.1016/j.phymed.2016.01.001
He, H., Zhuo, R., Dai, J., Wang, X., Huang, X., Wang, H., et al. (2019). Chelerythrine Induces Apoptosis via ROS-Mediated Endoplasmic Reticulum Stress and STAT3 Pathways in Human Renal Cell Carcinoma. J. Cell. Mol. Med. 24, 50–60. doi:10.1111/jcmm.14295
He, M., Yang, Z., Zhang, L., Song, C., Li, Y., and Zhang, X. (2017). Additive Effects of Cherlerythrine Chloride Combination with Erlotinib in Human Non-small Cell Lung Cancer Cells. PloS One 12, e0175466. doi:10.1371/journal.pone.0175466
He, N., Wang, P., Wang, P., Ma, C., and Kang, W. (2018). Antibacterial Mechanism of Chelerythrine Isolated from Root of Toddalia Asiatica (Linn) Lam. BMC Complement. Altern. Med. 18, 261. doi:10.1186/s12906-018-2317-3
He, X. P., and Ren, X. D. (1998). Protective Effects of Asiatic Toddalia Aqueous Extract on Ischemic Myocardium Induced by Pituitrin in Rats. Chin. J. Pathophysiol. 14, 283–286.
Hengsen, W. S., and Cheah, S. C. (2020). Chelerythrine Chloride Downregulates β-Catenin and Inhibits Stem Cell Properties of Non-small Cell Lung Carcinoma. Molecules 25, 224. doi:10.3390/molecules25010224
Herbert, J. M., Augereau, J. M., Gleye, J., and Maffrand, J. P. (1990). Chelerythrine Is a Potent and Specific Inhibitor of Protein Kinase C. Biochem. Biophys. Res. Commun. 172, 993–999. doi:10.1016/0006-291x(90)91544-3
Hoffmann, T. K., Leenen, K., Hafner, D., Balz, V., Gerharz, C. D., Grund, A., et al. (2002). Antitumor Activity of Protein Kinase C Inhibitors and Cisplatin in Human Head and Neck Squamous Cell Carcinoma Lines. Anticancer Drugs 13, 93–100. doi:10.1097/00001813-200201000-00011
Hotte, S. J., Oza, A., Winquist, E. W., Moore, M., Chen, E. X., Brown, S., et al. (2006). Phase I Trial of UCN-01 in Combination with Topotecan in Patients with Advanced Solid Cancers: a Princess Margaret Hospital Phase II Consortium Study. Ann. Oncol. 17, 334–340. doi:10.1093/annonc/mdj076
Hsieh, Y. S., Yang, S. F., Sethi, G., and Hu, D. N. (2015). Natural Bioactives in Cancer Treatment and Prevention. Biomed. Res. Int. 2015, 182835. doi:10.1155/2015/182835
Hu, N. X., Chen, M., Liu, Y. S., Shi, Q., Yang, B., Zhang, H. C., et al. (2019). Pharmacokinetics of Sanguinarine, Chelerythrine, and Their Metabolites in Broiler Chickens Following Oral and Intravenous Administration. J. Vet. Pharmacol. Ther. 42, 197–206. doi:10.1111/jvp.12729
Huang, C., Huang, Y., Zhang, Z., Liu, Y., and Liu, Z. (2021). Metabolism and Tissue Distribution of Chelerythrine and Effects of Macleaya Cordata Extracts on Liver NAD(P)H Quinone Oxidoreductase. Front. Veterinary Sci. 8–659771.
Inoue, M., Kishimoto, A., Takai, Y., and Nishizuka, Y. (1977). Studies on a Cyclic Nucleotide-independent Protein Kinase and its Proenzyme in Mammalian Tissues. II. Proenzyme and its Activation by Calcium-dependent Protease from Rat Brain. J. Biol. Chem. 252, 7610–7616. doi:10.1016/s0021-9258(17)41009-x
Irudayaraj, S. S., Sunil, C., Duraipandiyan, V., and Ignacimuthu, S. (2013). In Vitro antioxidant and Antihyperlipidemic Activities of Toddalia Asiatica (L) Lam. Leaves in Triton WR-1339 and High Fat Diet Induced Hyperlipidemic Rats. Food Chem. Toxicol. 60, 135–140. doi:10.1016/j.fct.2013.07.035
Iwasaki, H., Okabe, T., Takara, K., Toda, T., Shimatani, M., and Oku, H. (2010). Tumor-selective Cytotoxicity of Benzo[c]phenanthridine Derivatives from Toddalia Asiatica Lam. Cancer Chemother. Pharmacol. 65, 719–726. doi:10.1007/s00280-009-1077-7
Jana, J., Mondal, S., Bhattacharjee, P., Sengupta, P., Roychowdhury, T., Saha, P., et al. (2017). Chelerythrine Down Regulates Expression of VEGFA, BCL2 and KRAS by Arresting G-Quadruplex Structures at Their Promoter Regions. Sci. Rep. 7, 40706. doi:10.1038/srep40706
Jarić, S., Popović, Z., Macukanović-Jocić, M., Djurdjević, L., Mijatović, M., Karadzić, B., et al. (2007). An Ethnobotanical Study on the Usage of Wild Medicinal Herbs from Kopaonik Mountain (Central Serbia). J. Ethnopharmacol. 111, 160–175. doi:10.1016/j.jep.2006.11.007
Jermini, M., Dubois, J., Rodondi, P. Y., Zaman, K., Buclin, T., Csajka, C., et al. (2019). Complementary Medicine Use during Cancer Treatment and Potential Herb-Drug Interactions from a Cross-Sectional Study in an Academic Centre. Sci. Rep. 9, 5078. doi:10.1038/s41598-019-41532-3
Jin, Y., Khadka, D. B., and Cho, W. J. (2016). Pharmacological Effects of Berberine and its Derivatives: a Patent Update. Expert Opin. Ther. Pat. 26, 229–243. doi:10.1517/13543776.2016.1118060
Johnson, D. E., Burtness, B., Leemans, C. R., Lui, V. W. Y., Bauman, J. E., Grandis, J. R., et al. (2020). Head and Neck Squamous Cell Carcinoma. Nat. Rev. Dis. Prim. 6, 92. doi:10.1038/s41572-020-00224-3
Kaminskyy, V., Kulachkovskyy, O., and Stoika, R. (2008). A Decisive Role of Mitochondria in Defining Rate and Intensity of Apoptosis Induction by Different Alkaloids. Toxicol. Lett. 177, 168–181. doi:10.1016/j.toxlet.2008.01.009
Karunai Raj, M., Balachandran, C., Duraipandiyan, V., Agastian, P., and Ignacimuthu, S. (2012). Antimicrobial Activity of Ulopterol Isolated from Toddalia Asiatica (L.) Lam.: A Traditional Medicinal Plant. J. Ethnopharmacol. 140, 161–165. doi:10.1016/j.jep.2012.01.005
Kemény-Beke, A., Aradi, J., Damjanovich, J., Beck, Z., Facskó, A., Berta, A., et al. (2006). Apoptotic Response of Uveal Melanoma Cells upon Treatment with Chelidonine, Sanguinarine and Chelerythrine. Cancer Lett. 237, 67–75. doi:10.1016/j.canlet.2005.05.037
Kim, J., Kundu, M., Viollet, B., and Guan, K. L. (2011). AMPK and mTOR Regulate Autophagy through Direct Phosphorylation of Ulk1. Nat. Cell. Biol. 13, 132–141. doi:10.1038/ncb2152
Koleva, V. P., Dragoeva, A. P., Stoyanova, Z. D., Koynova, T., and Dashev, G. (2015). An Ethnobotanical Study on Current Status of Some Medicinal Plants Used in Bulgaria. Int. J. Curr. Microbiol. Appl. Sci. 4, 297–305.
Konopatskaya, O., and Poole, A. W. (2010). Protein Kinase Calpha: Disease Regulator and Therapeutic Target. Trends Pharmacol. Sci. 31, 8–14. doi:10.1016/j.tips.2009.10.006
Kortmansky, J., Shah, M. A., Kaubisch, A., Weyerbacher, A., Yi, S., Tong, W., et al. (2005). Phase I Trial of the Cyclin-dependent Kinase Inhibitor and Protein Kinase C Inhibitor 7-hydroxystaurosporine in Combination with Fluorouracil in Patients with Advanced Solid Tumors. J. Clin. Oncol. 23, 1875–1884. doi:10.1200/JCO.2005.03.116
Kosina, P., Gregorova, J., Gruz, J., Vacek, J., Kolar, M., Vogel, M., et al. (2010). Phytochemical and Antimicrobial Characterization of Macleaya Cordata Herb. Fitoterapia 81, 1006–1012. doi:10.1016/j.fitote.2010.06.020
Kosina, P., Walterová, D., Ulrichová, J., Lichnovský, V., Stiborová, M., Rýdlová, H., et al. (2003). Sanguinarine and Chelerythrine: Assessment of Safety on Pigs in Ninety Days Feeding Experiment. Food Chem. Toxicol. 42, 85–91. doi:10.1016/j.fct.2003.08.007
Kosiol, N., Juranek, S., Brossart, P., Heine, A., and Paeschke, K. (2021). G-quadruplexes: a Promising Target for Cancer Therapy. Mol. Cancer 20, 40. doi:10.1186/s12943-021-01328-4
Kujawska, M., Klepacki, P., and Łuczaj, Ł. (2017). Fischer's Plants in Folk Beliefs and Customs: a Previously Unknown Contribution to the Ethnobotany of the Polish-Lithuanian-Belarusian Borderland. J. Ethnobiol. Ethnomed 13, 20. doi:10.1186/s13002-017-0149-8
Kujawska, M., Uczaj, U., Sosnowska, J., and Klepacki, P. (2016). Roliny W Wierzeniach I Zwyczajach Ludowych. Sownik Adama Fischera. Wrocław: Polskie Towarzystwo Ludoznawcze. [Plants in folk beliefs and customs. Adam Fischer’s Lexicon].
Kumagai, M., Watanabe, A., Yoshida, I., Mishima, T., Nakamura, M., Nishikawa, K., et al. (2018). Evaluation of Aculeatin and Toddaculin Isolated from Toddalia Asiatica as Anti-inflammatory Agents in LPS-Stimulated RAW264 Macrophages. Biol. Pharm. Bull. 41, 132–137. doi:10.1248/bpb.b17-00607
Kumar, S., and Acharya, A. (2014). Chelerythrine Induces Reactive Oxygen Species-dependent Mitochondrial Apoptotic Pathway in a Murine T Cell Lymphoma. Tumour Biol. 35, 129–140. doi:10.1007/s13277-013-1016-4
Kumar, S., Deepak, P., Kumar, S., Gautam, P. K., and Acharya, A. (2013). A Benzophenanthridine Alkaloid, Chelerythrine Induces Apoptosis In Vitro in a Dalton's Lymphoma. J. Cancer Res. Ther. 9, 693–700. doi:10.4103/0973-1482.126485
Kumar, S., Tomar, M. S., and Acharya, A. (2015b). Activation of P53-dependent/-independent Pathways of Apoptotic Cell Death by Chelerythrine in a Murine T Cell Lymphoma. Leuk. Lymphoma 56, 1846–1855. doi:10.3109/10428194.2014.974042
Kumar, S., Tomar, M. S., and Acharya, A. (2015a). Chelerythrine Delayed Tumor Growth and Increased Survival Duration of Dalton's Lymphoma Bearing BALB/c H(2d) Mice by Activation of NK Cells In Vivo. J. Cancer Res. Ther. 11, 904–910. doi:10.4103/0973-1482.143342
Lee, H. J., Song, I. C., Yun, H. J., Jo, D. Y., and Kim, S. (2014). CXC Chemokines and Chemokine Receptors in Gastric Cancer: From Basic Findings towards Therapeutic Targeting. World J. Gastroenterol. 20, 1681–1693. doi:10.3748/wjg.v20.i7.1681
Lee, S., Rauch, J., and Kolch, W. (2020). Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity. Int. J. Mol. Sci. 21, 1102. doi:10.3390/ijms21031102
Lei, Y., Liu, L., Tang, X., Yang, D., Yang, X., and He, F. (2018). Sanguinarine and Chelerythrine: Two Natural Products for Mitochondria-Imaging with Aggregation-Induced Emission Enhancement and pH-Sensitive Characteristics. RSC Adv. 8, 3919–3927. doi:10.1039/c7ra12920c
Leitges, M. (2007). Functional PKC In Vivo Analysis Using Deficient Mouse Models. Biochem. Soc. Trans. 35, 1018–1020. doi:10.1042/BST0351018
Levine, B., and Kroemer, G. (2008). Autophagy in the Pathogenesis of Disease. Cell. 132, 27–42. doi:10.1016/j.cell.2007.12.018
Li, J., Li, B., Wu, Y., Shuang, S., Dong, C., and Choi, M. M. (2012). Luminescence and Binding Properties of Two Isoquinoline Alkaloids Chelerythrine and Sanguinarine with ctDNA. Spectrochim. Acta A Mol. Biomol. Spectrosc. 95, 80–85. doi:10.1016/j.saa.2012.04.075
Li, T., Chen, X., Dai, X. Y., Wei, B., Weng, Q. J., Chen, X., et al. (2017). Novel Hsp90 Inhibitor Platycodin D Disrupts Hsp90/Cdc37 Complex and Enhances the Anticancer Effect of mTOR Inhibitor. Toxicol. Appl. Pharmacol. 330, 65–73. doi:10.1016/j.taap.2017.07.006
Li, W., Xing, W., Niu, X., Zhou, P., and Fan, T. (2013a). The Pharmacokinetics of Chelerythrine Solution and Chelerythrine Liposomes after Oral Administration to Rats. Planta Med. 79, 654–660. doi:10.1055/s-0032-1328540
Li, W., Xing, W., Niu, X., Zhou, P., and Fan, T. (2013b). The Pharmacokinetics of Chelerythrine Solution and Chelerythrine Liposomes after Oral Administration to Rats. Planta Med. 79, 654–660. doi:10.1055/s-0032-1328540
Li, W.-F., Hao, D.-J., Fan, T., Huang, H.-M., Yao, H., and Niu, X.-F. (2014). Protective Effect of Chelerythrine against Ethanol-Induced Gastric Ulcer in Mice. Chemico-Biological Interact. 208, 18–27. doi:10.1016/j.cbi.2013.11.011
Li, X., Qiu, Z., Jin, Q., Chen, G., and Guo, M. (2018). Cell Cycle Arrest and Apoptosis in HT-29 Cells Induced by Dichloromethane Fraction from Toddalia Asiatica (L.) Lam. Front. Pharmacol. 9, 629. doi:10.3389/fphar.2018.00629
Li, X. L., Yao, J. Y., Zhou, Z. M., Shen, J. Y., Ru, H. S., and Liu, X. L. (2011). Activity of the Chelerythrine, a Quaternary Benzo[c]phenanthridine Alkaloid from Chelidonium Majus L. On Dactylogyrus Intermedius. Parasitol. Res. 109, 247–252. doi:10.1007/s00436-011-2320-9
Lin, L., Liu, Y. C., Huang, J. L., Liu, X. B., Qing, Z. X., Zeng, J. G., et al. (2018). Medicinal Plants of the Genus Macleaya (Macleaya Cordata, Macleaya Microcarpa): A Review of Their Phytochemistry, Pharmacology, and Toxicology. Phytother. Res. 32, 19–48. doi:10.1002/ptr.5952
Lin, Q., Pu, H., Guan, H., Ma, C., Zhang, Y., Ding, W., et al. (2020). Rapid Identification and Pharmacokinetic Studies of Multiple Active Alkaloids in Rat Plasma through UPLC-Q-TOF-MS and UPLC-MS/MS after the Oral Administration of Zanthoxylum Nitidum Extract. J. Pharm. Biomed. Anal. 186, 113232. doi:10.1016/j.jpba.2020.113232
Lin, W., Huang, J., Yuan, Z., Feng, S., Xie, Y., and Ma, W. (2017). Protein Kinase C Inhibitor Chelerythrine Selectively Inhibits Proliferation of Triple-Negative Breast Cancer Cells. Sci. Rep. 7, 2022. doi:10.1038/s41598-017-02222-0
Liu, M., Lin, Y. L., Chen, X. R., Liao, C. C., and Poo, W. K. (2013). In Vitro assessment of Macleaya Cordata Crude Extract Bioactivity and Anticancer Properties in Normal and Cancerous Human Lung Cells. Exp. Toxicol. Pathol. 65, 775–787. doi:10.1016/j.etp.2012.11.004
Liu, X., Liu, C., Guan, N., and Liu, J. (2017). Long Term Toxicity of Chelerythrine on NF-Κb Expression in Rat Pulmonary Tissues. Int. J. Clin. Exp. Med. 10, 11464–11471.
Luo, Y. M. (1987). Macleaya Cordata Is Main External Treatment of Skin Diseases. Fujian J. Traditional Chineses Med. 66.
Ma, C. X., Ellis, M. J., Petroni, G. R., Guo, Z., Cai, S. R., Ryan, C. E., et al. (2013). A Phase II Study of UCN-01 in Combination with Irinotecan in Patients with Metastatic Triple Negative Breast Cancer. Breast Cancer Res. Treat. 137, 483–492. doi:10.1007/s10549-012-2378-9
Mah, L. Y., and Ryan, K. M. (2011). Autophagy and Cancer. Cold Spring Harb. Perspect. Biol. 4, a008821. doi:10.1101/cshperspect.a008821
Maji, A. K., Banerji, P., and Banerji, P. (2015). Chelidonium Majus L. (Greater Celandine) - A Review on its Phytochemical and Therapeutic Perspectives. Int. J. Herb. Med. 3, 10–27. doi:10.22271/flora.2015.v3.i1.03
Malikova, J., Zdarilova, A., and Hlobilkova, A. (2006). Effects of Sanguinarine and Chelerythrine on the Cell Cycle and Apoptosis. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 150, 5–12. doi:10.5507/bp.2006.001
Malíková, J., Zdarilová, A., Hlobilková, A., and Ulrichová, J. (2006). The Effect of Chelerythrine on Cell Growth, Apoptosis, and Cell Cycle in Human Normal and Cancer Cells in Comparison with Sanguinarine. Cell. Biol. Toxicol. 22, 439–453.
Mamedov, N., Gardner, Z., and Craker, L. E. (2005). Medicinal Plants Used in Russia and Central Asia for the Treatment of Selected Skin Conditions. J. Herbs, Spices Med. Plants 11, 191–222. doi:10.1300/j044v11n01_07
Manna, F. L., Karkampouna, S., Zoni, E., De Menna, M., Hensel, J., Thalmann, G. N., et al. (2019). Metastases in Prostate Cancer. Cold Spring Harb. Perspect. Med. 9, a033688. doi:10.1101/cshperspect.a033688
Martin, K. R., and Barrett, J. C. (2002). Reactive Oxygen Species as Double-Edged Swords in Cellular Processes: Low-Dose Cell Signaling versus High-Dose Toxicity. Hum. Exp. Toxicol. 21, 71–75. doi:10.1191/0960327102ht213oa
Matkar, S. S., Wrischnik, L. A., and Hellmann-Blumberg, U. (2008). Production of Hydrogen Peroxide and Redox Cycling Can Explain How Sanguinarine and Chelerythrine Induce Rapid Apoptosis. Arch. Biochem. Biophys. 477, 43–52. doi:10.1016/j.abb.2008.05.019
MayerGottfried, J., Uehleke, Bernhard., and Saum, Kilian. (2002). Handbuch der Klosterheilkunde. RM Buch und Medienvertrieb.
Medvetz, D., Priolo, C., and Henske, E. P. (2014). Therapeutic Targeting of Cellular Metabolism in Cells with Hyperactive mTORC1: A Paradigm Shift. Mol. Cancer Res. 13, 3–8. doi:10.1158/1541-7786.MCR-14-0343
Melling, C. W., Thorp, D. B., Milne, K. J., and Noble, E. G. (2009). Myocardial Hsp70 Phosphorylation and PKC-Mediated Cardioprotection Following Exercise. Cell. Stress Chaperones 14, 141–150. doi:10.1007/s12192-008-0065-x
Menković, N., Savikin, K., Tasić, S., Zdunić, G., Stesević, D., Milosavljević, S., et al. (2011). Ethnobotanical Study on Traditional Uses of Wild Medicinal Plants in Prokletije Mountains (Montenegro). J. Ethnopharmacol. 133, 97–107. doi:10.1016/j.jep.2010.09.008
Milkovic, L., Cipak Gasparovic, A., Cindric, M., Mouthuy, P. A., and Zarkovic, N. (2019). Short Overview of ROS as Cell Function Regulators and Their Implications in Therapy Concepts. Cells 8, 793. doi:10.3390/cells8080793
Millimouno, F. M., Dong, J., Yang, L., Li, J., and Li, X. (2014). Targeting Apoptosis Pathways in Cancer and Perspectives with Natural Compounds from Mother Nature. Cancer Prev. Res. (Phila) 7, 1081–1107. doi:10.1158/1940-6207.CAPR-14-0136
Mueller, H., Liu, R., David, F., and Eppenberger, U. (1997). Selective Modulation of Protein Kinase A and Protein Kinase C Activities in Epidermal Growth Factor (EGF)-Stimulated MCF-7 Breast Cancer Cells. Biol. Chem. 378, 1023–1029. doi:10.1515/bchm.1997.378.9.1023
Newman, D. J., and Cragg, G. M. (2016). Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 79, 629–661. doi:10.1021/acs.jnatprod.5b01055
Newman, D. J., Cragg, G. M., and Snader, K. M. (2003). Natural Products as Sources of New Drugs over the Period 1981-2002. J. Nat. Prod. 66, 1022–1037. doi:10.1021/np030096l
Ngarivhume, T., Van't Klooster, C. I., de Jong, J. T., and Van der Westhuizen, J. H. (2015). Medicinal Plants Used by Traditional Healers for the Treatment of Malaria in the Chipinge District in Zimbabwe. J. Ethnopharmacol. 159, 224–237. doi:10.1016/j.jep.2014.11.011
Ni, H., Martínez, Y., Guan, G., Rodríguez, R., Más, D., Peng, H., et al. (20162016). Analysis of the Impact of Isoquinoline Alkaloids, Derived from Macleaya Cordata Extract, on the Development and Innate Immune Response in Swine and Poultry. Biomed. Res. Int. 2016, 1352146. doi:10.1155/2016/1352146
Ni, J., Zhao, Y., Su, J., Liu, Z., Fang, S., Li, L., et al. (2020). Toddalolactone Protects Lipopolysaccharide-Induced Sepsis and Attenuates Lipopolysaccharide-Induced Inflammatory Response by Modulating HMGB1-NF-Κb Translocation. Front. Pharmacol. 11, 109. doi:10.3389/fphar.2020.00109
Niu, X. F., Zhou, P., Li, W. F., and Xu, H. B. (2011). Effects of Chelerythrine, a Specific Inhibitor of Cyclooxygenase-2, on Acute Inflammation in Mice. Fitoterapia 82, 620–625. doi:10.1016/j.fitote.2011.01.020
Noureini, S. K., Esmaeili, H., Abachi, F., Khiali, S., Islam, B., Kuta, M., et al. (2017). Selectivity of Major Isoquinoline Alkaloids from Chelidonium Majus towards Telomeric G-Quadruplex: A Study Using a Transition-FRET (T-FRET) Assay. Biochim. Biophys. Acta Gen. Subj. 1861, 2020–2030. doi:10.1016/j.bbagen.2017.05.002
Novick, A. C. (2007). Kidney Cancer: Past, Present, and Future. Urol. Oncol. 25, 188–195. doi:10.1016/j.urolonc.2007.03.006
Orwa, J. A., Jondiko, I. J., Minja, R. J., and Bekunda, M. (2008). The Use of Toddalia Asiatica (L) Lam. (Rutaceae) in Traditional Medicine Practice in East Africa. J. Ethnopharmacol. 115, 257–262. doi:10.1016/j.jep.2007.09.024
Orwa, J. A., Ngeny, L., Mwikwabe, N. M., Ondicho, J., and Jondiko, I. J. (2013). Antimalarial and Safety Evaluation of Extracts from Toddalia Asiatica (L) Lam. (Rutaceae). J. Ethnopharmacol. 145, 587–590. doi:10.1016/j.jep.2012.11.034
Otto, T., and Sicinski, P. (2017). Cell Cycle Proteins as Promising Targets in Cancer Therapy. Nat. Rev. Cancer 17, 93–115. doi:10.1038/nrc.2016.138
Park, S. W., Kim, S. R., Kim, Y., Lee, J. H., Woo, H. J., Yoon, Y. K., et al. (2015). Chelidonium Majus L. Extract Induces Apoptosis through Caspase Activity via MAPK-independent NF-Κb Signaling in Human Epidermoid Carcinoma A431 Cells. Oncol. Rep. 33, 419–424. doi:10.3892/or.2014.3566
Pelicano, H., Carney, D., and Huang, P. (2004). ROS Stress in Cancer Cells and Therapeutic Implications. Drug Resist Updat 7, 97–110. doi:10.1016/j.drup.2004.01.004
Platzbecker, U., Ward, J. L., and Deeg, H. J. (2003). Chelerythrin Activates Caspase-8, Downregulates FLIP Long and Short, and Overcomes Resistance to Tumour Necrosis Factor-Related Apoptosis-Inducing Ligand in KG1a Cells. Br. J. Haematol. 122, 489–497. doi:10.1046/j.1365-2141.2003.04445.x
Pradhan, A. B., Bhuiya, S., Haque, L., and Das, S. (2017). Spectroscopic Study on the Binding of Chelerythrine with Duplex Poly (rA): A Model of RNA Intercalation. Int. J. Biol. Macromol. 95, 340–347. doi:10.1016/j.ijbiomac.2016.11.073
Qiu, J., Zhu, M., Wang, Y., Chen, B., Bai, R., Chen, F., et al. (2022). Pharmacokinetic and Excretion Study of Eight Active Constituents in Rat by LC-MS/MS after Oral Administration of the Toddalia Asiatica Extract. Anal. Biochem. 640, 114407. doi:10.1016/j.ab.2021.114407
Ren, Y., de Blanco, E. J. C., Fuchs, J. R., Soejarto, D. D., Burdette, J. E., Swanson, S. M., et al. (2019). Potential Anticancer Agents Characterized from Selected Tropical Plants. J. Nat. Prod. 82, 657–679. doi:10.1021/acs.jnatprod.9b00018
Ryu, S., Lim, W., Bazer, F. W., and Song, G. (2017). Chrysin Induces Death of Prostate Cancer Cells by Inducing ROS and ER Stress. J. Cell. Physiol. 232, 3786–3797. doi:10.1002/jcp.25861
Sai, C., Qin, W., Meng, J., Gao, L. N., Huang, L., Zhang, Z., et al. (2021). Macleayins A from Macleaya Promotes Cell Apoptosis through Wnt/β-Catenin Signaling Pathway and Inhibits Proliferation, Migration, and Invasion in Cervical Cancer HeLa Cells. Front. Pharmacol. 12, 668348. doi:10.3389/fphar.2021.668348
Sausville, E. A., Arbuck, S. G., Messmann, R., Headlee, D., Bauer, K. S., Lush, R. M., et al. (2001). Phase I Trial of 72-hour Continuous Infusion UCN-01 in Patients with Refractory Neoplasms. J. Clin. Oncol. 19, 2319–2333. doi:10.1200/JCO.2001.19.8.2319
Savikin, K., Zdunić, G., Menković, N., Zivković, J., Cujić, N., Tereščenko, M., et al. (2013). Ethnobotanical Study on Traditional Use of Medicinal Plants in South-Western Serbia, Zlatibor District. J. Ethnopharmacol. 146, 803–810. doi:10.1016/j.jep.2013.02.006
Schlachterman, A. W. W. C., Craft, W. W., Hilgenfeldt, E., Mitra, A., and Cabrera, R. (2015). Current and Future Treatments for Hepatocellular Carcinoma. World J. Gastroenterol. 21, 8478–8491. doi:10.3748/wjg.v21.i28.8478
Scorilas, A., Karameris, A., Arnogiannaki, N., Ardavanis, A., Bassilopoulos, P., Trangas, T., et al. (2001). Overexpression of Matrix-Metalloproteinase-9 in Human Breast Cancer: a Potential Favourable Indicator in Node-Negative Patients. Br. J. Cancer 84, 1488–1496. doi:10.1054/bjoc.2001.1810
Sedo, A., Vlasicová, K., Barták, P., Vespalec, R., Vicar, J., Simánek, V., et al. (2002). Quaternary Benzo[c]phenanthridine Alkaloids as Inhibitors of Aminopeptidase N and Dipeptidyl Peptidase IV. Phytother. Res. 16, 84–87. doi:10.1002/ptr.969
Shanmugam, M. K., Warrier, S., Kumar, A. P., Sethi, G., and Arfuso, F. (2017). Potential Role of Natural Compounds as Anti-angiogenic Agents in Cancer. Curr. Vasc. Pharmacol. 15, 503–519. doi:10.2174/1570161115666170713094319
Shemon, A. N., Sluyter, R., Conigrave, A. D., and Wiley, J. S. (2004). Chelerythrine and Other Benzophenanthridine Alkaloids Block the Human P2X7 Receptor. Br. J. Pharmacol. 142, 1015–1019. doi:10.1038/sj.bjp.0705868
Shen, L. X., Liu, G. F., Song, J. S., Cao, Y. H., Peng, X., Wu, R. R., et al. (2022). Sex Differences in the Pharmacokinetics and Tissue Residues of Macleaya Cordata Extracts in Rats. Xenobiotica 52, 46–53. doi:10.1080/00498254.2022.2048323
Shi, L., Li, D., and Kang, W. Y. (2011). Research Progress of Chemical Constituents and Pharmacological Effects of Toddalia Asiatica. China Pharm. 22, 666–668.
Si, Y., Wang, J., Liu, X., Zhou, T., Xiang, Y., Zhang, T., et al. (2020). Ethoxysanguinarine, a Novel Direct Activator of AMP-Activated Protein Kinase, Induces Autophagy and Exhibits Therapeutic Potential in Breast Cancer Cells. Front. Pharmacol. 10, 1503. doi:10.3389/fphar.2019.01503
Siomboing, X., Gressier, B., Dine, T., Brunet, C., Luyckx, M., Cazin, M., et al. (2001). Investigation of the Inhibitory Effects of Chelerythrine Chloride on the Translocation of the Protein Kinase C βI, βII, ζ in Human Neutrophils. Il Farm. 56, 859–865. doi:10.1016/s0014-827x(01)01165-x
Stiborová, M., Simánek, V., Frei, E., Hobza, P., and Ulrichová, J. (2002). DNA Adduct Formation from Quaternary Benzo[c]phenanthridine Alkaloids Sanguinarine and Chelerythrine as Revealed by the 32P-Postlabeling Technique. Chemico-Biological Interact. 140, 231–242.
Sun, X., Wang, Q., Wang, Y., Du, L., Xu, C., and Liu, Q. (2016). Brusatol Enhances the Radiosensitivity of A549 Cells by Promoting ROS Production and Enhancing DNA Damage. Int. J. Mol. Sci. 17, 997. doi:10.3390/ijms17070997
Tang, Z. H., Cao, W. X., Wang, Z. Y., Lu, J. H., Liu, B., Chen, X., et al. (2017). Induction of Reactive Oxygen Species-Stimulated Distinctive Autophagy by Chelerythrine in Non-small Cell Lung Cancer Cells. Redox Biol. 12, 367–376. doi:10.1016/j.redox.2017.03.009
Tanida, I., Ueno, T., and Kominami, E. (2008). LC3 and Autophagy. Methods Mol. Biol. 445, 77–88. doi:10.1007/978-1-59745-157-4_4
Terenzi, A., Gattuso, H., Spinello, A., Keppler, B. K., Chipot, C., Dehez, F., et al. (2019). Targeting G-Quadruplexes with Organic Dyes: Chelerythrine-DNA Binding Elucidated by Combining Molecular Modeling and Optical Spectroscopy. Antioxidants (Basel) 8, 472. doi:10.3390/antiox8100472
Tsujimoto, Y., and Shimizu, S. (2005). Another way to die: autophagic programmed cell death. Cell. Death Differ. 12 Suppl 2, 1528–1534. doi:10.1038/sj.cdd.4401777
Tuzimski, T., Petruczynik, A., Plech, T., Kaproń, B., Makuch-Kocka, A., Szultka-Młyńska, M., et al. (2021). Determination of Cytotoxic Activity of Sanguinaria Canadensis Extracts against Human Melanoma Cells and Comparison of Their Cytotoxicity with Cytotoxicity of Some Anticancer Drugs. Molecules 26, 1738. doi:10.3390/molecules26061738
Uglyanitsa, K. N., Nefyodov, L. I., Karayedova, L. M., Nowicky, J. W., and Brzosko, W. (2000). Clinical Aspects of Cancer Treatment and New Biochemical Mechanisms of the Drug Ukrain. Drugs Exp. Clin. Res. 26, 239–247.
Ulrichová, J., Dvořák, Z., Vičar, J., Lata, J., Smržová, J., Šedo, A., et al. (2001). Cytotoxicity of Natural Compounds in Hepatocyte Cell Culture Models. The Case of Quaternary Benzo[c]phenanthridine Alkaloids. Toxicol. Lett. 125, 125–132.
Ulrichová, J., Walterová, D., Vavrečková, C., Kamarád, Vojtěch., and Šimánek, V. (1996). Cytotoxicity of Benzo[c]phenanthridinium Alkaloids in Isolated Rat Hepatocytes. Phytotherapy Res. 10, 220–223.
Urbanová, J., Lubal, P., Slaninová, I., Táborská, E., and Táborský, P. (2009). Fluorescence Properties of Selected Benzo[c]phenantridine Alkaloids and Studies of Their Interaction with CT DNA. Anal. Bioanal. Chem. 394, 997–1002.
Valastyan, S., and Weinberg, R. A. (2011). Tumor Metastasis: Molecular Insights and Evolving Paradigms. Cell. 147, 275–292. doi:10.1016/j.cell.2011.09.024
Varshney, D., Spiegel, J., Zyner, K., Tannahill, D., and Balasubramanian, S. (2020). The Regulation and Functions of DNA and RNA G-Quadruplexes. Nat. Rev. Mol. Cell. Biol. 21, 459–474. doi:10.1038/s41580-020-0236-x
Vieira, S. M., de Oliveira, V. H., Valente, Rdo. C., Moreira, Oda. C., Fontes, C. F., and Mignaco, J. A. (2015). Chelerythrine Inhibits the Sarco/endoplasmic Reticulum Ca(2+)-ATPase and Results in Cell Ca(2+) Imbalance. Arch. Biochem. Biophys. 570, 58–65. doi:10.1016/j.abb.2015.02.019
Vrba, J., Dolezel, P., Vicar, J., Modrianský, M., and Ulrichová, J. (2008). Chelerythrine and Dihydrochelerythrine Induce G1 Phase Arrest and Bimodal Cell Death in Human Leukemia HL-60 Cells. Toxicol Vitro 22, 1008–1017. doi:10.1016/j.tiv.2008.02.007
Wan, X., Min, Y., Bludau, H., Keith, A., Sheiko, S. S., Jordan, R., et al. (2018). Drug Combination Synergy in Worm-like Polymeric Micelles Improves Treatment Outcome for Small Cell and Non-small Cell Lung Cancer. Acs Nano 12, 2426–2439. doi:10.1021/acsnano.7b07878
Wang, J., Song, Y., Zhang, N., Li, N., Liu, C., and Wang, B. (2021). Using Liposomes to Alleviate the Toxicity of Chelerythrine, a Natural PKC Inhibitor, in Treating Non-small Cell Lung Cancer. Front. Oncol. 11, 658543. doi:10.3389/fonc.2021.658543
Wang, P. Q., Yin, Z. H., and Kang, W. Y. (2013). Advance in Studies on Pharmacological Activities of Chelerythrine. Zhongguo Zhong Yao Za Zhi 38, 2745–2749.
Warowicka, A., Popenda, Ł., Bartkowiak, G., Musidlak, O., Litowczenko-Cybulska, J., Kuźma, D., et al. (2019). Protoberberine Compounds Extracted from Chelidonium Majus L. As Novel Natural Photosensitizers for Cancer Therapy. Phytomedicine 64, 152919. doi:10.1016/j.phymed.2019.152919
Watanabe, A., Kumagai, M., Mishima, T., Ito, J., Otoki, Y., Harada, T., et al. (2015). Toddaculin, Isolated from of Toddalia Asiatica (L.) Lam., Inhibited Osteoclastogenesis in RAW 264 Cells and Enhanced Osteoblastogenesis in MC3T3-E1 Cells. PloS One 10, e0127158. doi:10.1371/journal.pone.0127158
Wei, Q., Zhao, M., and Li, X. (2017). Extraction of Chelerythrine and its Effects on Pathogenic Fungus Spore Germination. Pharmacogn. Mag. 13, 600–606. doi:10.4103/pm.pm_545_16
Wong, R. S. (2011). Apoptosis in Cancer: from Pathogenesis to Treatment. J. Exp. Clin. Cancer Res. 30, 87. doi:10.1186/1756-9966-30-87
Wu, M. W., and Zhu, J. H. (2002). Pharmacological Research and Application of Macleaya Cordata. Prim. J. Chin. Mater Med., 46–48.
Wu, S., Yang, Y., Li, F., Huang, L., Han, Z., Wang, G., et al. (2018). Chelerythrine Induced Cell Death through ROS-dependent ER Stress in Human Prostate Cancer Cells. Onco Targets Ther. 11, 2593–2601. doi:10.2147/OTT.S157707
Xia, Q., and Liu, Y. (2007). Pharmacognosy Identification of Ethno-Medicine Toddalia asiatica(L.)Lam. Journal of Southwest University for Nationalities(Natural Science Edition) 33 (5), 1101–1103. doi:10.3969/j.issn.1003-2843.2007.05.025
Xie, H., Yang, J., Feng, S., Cheng, P., Zeng, J., and Xiong, X. (2015). Simultaneous Quantitative Determination of Sanguinarine, Chelerythrine, Dihydrosanguinarine and Dihydrochelerythrine in Chicken by HPLC-MS/MS Method and its Applications to Drug Residue and Pharmacokinetic Study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 985, 124–130. doi:10.1016/j.jchromb.2015.01.001
Xu, X., Wang, Y., Chen, Z., Sternlicht, M. D., Hidalgo, M., and Steffensen, B. (2005). Matrix Metalloproteinase-2 Contributes to Cancer Cell Migration on Collagen. Cancer Res. 65, 130–136.
Yang, B., Zhang, D., Qian, J., and Cheng, Y. (2020). Chelerythrine Suppresses Proliferation and Metastasis of Human Prostate Cancer Cells via Modulating MMP/TIMP/NF-κB System. Mol. Cell. Biochem. 474, 199–208. doi:10.1007/s11010-020-03845-0
Yang, K., Tong, L., Chen, C., Zhang, P., Pi, H., Ruan, H., et al. (2013). Therapeutic Effects of Extracts from Radix Toddaliae Asiaticae on Collagen-Induced Arthritis in Balb/c Mice. J. Ethnopharmacol. 146, 355–362. doi:10.1016/j.jep.2013.01.004
Yang, R., Piperdi, S., and Gorlick, R. (2008). Activation of the RAF/mitogen-activated Protein/extracellular Signal-Regulated Kinase Kinase/extracellular Signal-Regulated Kinase Pathway Mediates Apoptosis Induced by Chelerythrine in Osteosarcoma. Clin. Cancer Res. 14, 6396–6404. doi:10.1158/1078-0432.CCR-07-5113
Yang, S. F., Weng, C. J., Sethi, G., and Hu, D. N. (2013). Natural Bioactives and Phytochemicals Serve in Cancer Treatment and Prevention. Evid. Based Complement. Altern. Med. 2013, 698190. doi:10.1155/2013/698190
Yang, T., Xu, R., Su, Q., Wang, H., Liu, F., Dai, B., et al. (2020). Chelerythrine Hydrochloride Inhibits Proliferation and Induces Mitochondrial Apoptosis in Cervical Cancer Cells via PI3K/BAD Signaling Pathway. Toxicol Vitro 68, 104965. doi:10.1016/j.tiv.2020.104965
Yang, Z. J., Chee, C. E., Huang, S., and Sinicrope, F. A. (2011). The Role of Autophagy in Cancer: Therapeutic Implications. Mol. Cancer Ther. 10, 1533–1541. doi:10.1158/1535-7163.MCT-11-0047
Yu, R., Mandlekar, S., Tan, T. H., and Kong, A. N. (2000). Activation of P38 and C-Jun N-Terminal Kinase Pathways and Induction of Apoptosis by Chelerythrine Do Not Require Inhibition of Protein Kinase C. J. Biol. Chem. 275, 9612–9619. doi:10.1074/jbc.275.13.9612
Zdarilová, A., Vrzal, R., Rypka, M., Ulrichová, J., and Dvorák, Z. (2006). Investigation of Sanguinarine and Chelerythrine Effects on CYP1A1 Expression and Activity in Human Hepatoma Cells. Food Chem. Toxicol. 44, 242–249.
Zeng, Z., Tian, R., Feng, J., Yang, N. A., and Yuan, L. (2021). A Systematic Review on Traditional Medicine Toddalia Asiatica (L.) Lam.: Chemistry and Medicinal Potential. Saudi Pharm. J. 29, 781–798. doi:10.1016/j.jsps.2021.05.003
Zhang, B., Wu, Q., Ye, X. F., Liu, S., Lin, X. F., and Chen, M. C. (2003). Roles of PLC-Gamma2 and PKCalpha in TPA-Induced Apoptosis of Gastric Cancer Cells. World J. Gastroenterol. 9, 2413–2418. doi:10.3748/wjg.v9.i11.2413
Zhang, F., Chen, B., Xiao, S., and Yao, S.-z. (2005). Optimization and Comparison of Different Extraction Techniques for Sanguinarine and Chelerythrine in Fruits of Macleaya Cordata (Willd) R. Br. Sep. Purif. Technol. 42, 283–290. doi:10.1016/j.seppur.2004.09.002
Zhang, Y. H., Bhunia, A., Wan, K. F., Lee, M. C., Chan, S. L., Yu, V. C., et al. (2006). Chelerythrine and Sanguinarine Dock at Distinct Sites on BclXL that Are Not the Classic BH3 Binding Cleft. J. Mol. Biol. 364, 536–549. doi:10.1016/j.jmb.2006.09.023
Zhang, Z. F., Guo, Y., Zhang, J. B., and Wei, X. H. (2011). Induction of Apoptosis by Chelerythrine Chloride through Mitochondrial Pathway and Bcl-2 Family Proteins in Human Hepatoma SMMC-7721 Cell. Arch. Pharm. Res. 34, 791–800. doi:10.1007/s12272-011-0513-5
Zhang, Z., Guo, Y., Zhang, L., Zhang, J., and Wei, X. (2012). Chelerythrine Chloride from Macleaya Cordata Induces Growth Inhibition and Apoptosis in Human Gastric Cancer BGC-823 Cells. Acta Pharm. Sin. B 2, 464–471. doi:10.1016/j.apsb.2011.12.013
Zhao, N. J., Wang, L. L., Liu, Z. Y., Wang, Q., Liu, L., Sun, Z. L., et al. (2021). Pharmacokinetics of Chelerythrine and its Metabolite after Oral and Intramuscular Administrations in Pigs. Xenobiotica 51, 1264–1270. doi:10.1080/00498254.2021.1882714
Zheng, W., Qiu, L., Wang, R., Feng, X., Han, Y., Zhu, Y., et al. (2015). Selective Targeting of PPARγ by the Natural Product Chelerythrine with a Unique Binding Mode and Improved Antidiabetic Potency. Sci. Rep. 5, 12222. doi:10.1038/srep12222
Zheng, X., Ryan, A., Patel, N., Klemons, S., Hansson, A., Shih, W. J., et al. (2005). Synergistic Stimulatory Effect of 12-O-Tetradecanoylphorbol-13-Acetate and Capsaicin on Macrophage Differentiation in HL-60 and HL-525 Human Myeloid Leukemia Cells. Int. J. Oncol. 26, 441–448. doi:10.3892/ijo.26.2.441
Zhou, Q., Liu, Y., Wang, X., and Di, X. (2013). A Sensitive and Selective Liquid Chromatography-Tandem Mass Spectrometry Method for Simultaneous Determination of Five Isoquinoline Alkaloids from Chelidonium Majus L. In Rat Plasma and its Application to a Pharmacokinetic Study. J. Mass Spectrom. 48, 111–118. doi:10.1002/jms.3133
Zhu, G. H., Wong, B. C., Eggo, M. C., Yuen, S. T., Lai, K. C., and Lam, S. K. (1999). Pharmacological Inhibition of Protein Kinase C Activity Could Induce Apoptosis in Gastric Cancer Cells by Differential Regulation of Apoptosis-Related Genes. Dig. Dis. Sci. 44, 2020–2026. doi:10.1023/a:1026670301787
Zhu, Y., Pan, Y., Zhang, G., Wu, Y., Zhong, W., Chu, C., et al. (2018). Chelerythrine Inhibits Human Hepatocellular Carcinoma Metastasis In Vitro. Biol. Pharm. Bull. 41, 36–46. doi:10.1248/bpb.b17-00451
Zielińska, S., Jezierska-Domaradzka, A., Wójciak-Kosior, M., Sowa, I., Junka, A., and Matkowski, A. M. (2018). Greater Celandine's Ups and Downs-21 Centuries of Medicinal Uses of Chelidonium Majus from the Viewpoint of Today's Pharmacology. Front. Pharmacol. 9, 299. doi:10.3389/fphar.2018.00299
Keywords: chelerythrine, traditional botanical drugs, anticancer, molecular mechanism, future direction
Citation: Chen N, Qi Y, Ma X, Xiao X, Liu Q, Xia T, Xiang J, Zeng J and Tang J (2022) Rediscovery of Traditional Plant Medicine: An Underestimated Anticancer Drug of Chelerythrine. Front. Pharmacol. 13:906301. doi: 10.3389/fphar.2022.906301
Received: 28 March 2022; Accepted: 26 April 2022;
Published: 01 June 2022.
Edited by:
Shuai Ji, Xuzhou Medical University, ChinaReviewed by:
Tanaya Roychowdhury, Indian Institute of Chemical Biology (CSIR), IndiaRudradip Pattanayak, University of Alabama at Birmingham, United States
Lina Wen, Capital Medical University, China
Copyright © 2022 Chen, Qi, Ma, Xiao, Liu, Xia, Xiang, Zeng and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinhao Zeng, zengjinhao@cdutcm.edu.cn; Jianyuan Tang, tangjy@cdutcm.edu.cn
†These authors have contributed equally to this work
 Nianzhi Chen
Nianzhi Chen Yulin Qi
Yulin Qi Xiao Ma
Xiao Ma Xiaolin Xiao
Xiaolin Xiao Qingsong Liu
Qingsong Liu Ting Xia
Ting Xia Juyi Xiang
Juyi Xiang Jinhao Zeng
Jinhao Zeng Jianyuan Tang
Jianyuan Tang