Pretreatment and Bioconversion for Valorization of Residues of Non-Edible Oilseeds
Abstract
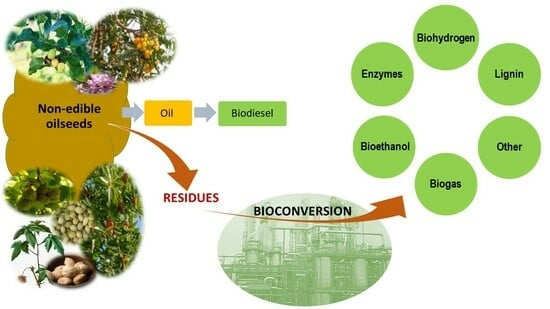
:1. Introduction
2. Vegetable Oils—From Traditional Edible Sources to the Emerging Use of Non-Edible Oilseeds
3. Residues of Processing of Non-Edible Oilseeds
4. Valorization of Non-Edible Oilseed Residues
4.1. Bioconversion Processes for Valorization of Non-Edible Oilseed Residues
4.1.1. Anaerobic Digestion
| Material | Conditions | Results | Ref. |
|---|---|---|---|
| Jatropha seed cake | AD of a 1:20 cake/water slurry in a 5-L batch reactor at 30 °C for 60 days. | Methane yield: 156 L/kg of seed cake; COD removal: 52%. | [91] |
| Jatropha seed cake | Semi-continuous flow at 30 °C; COD range: 1.25–5 kg/m3 day. | Highest methane yield (340 L/kg COD degraded) was obtained at an OLR of 1.25 kg COD/m3 day. | [92] |
| Jatropha seed cake | AD of cow dung alone and mixed with jatropha cake in 2-L plastic jars for 40 days. | Biogas yield of jatropha cake (0.170 m3/kg) was higher than that of cow dung (0.166 m3/kg). The digestate was a suitable fertilizer for maize and tomato. | [93] |
| Jatropha seed cake | Jatropha cake alone or combined with cattle dung, 37 °C, 5-L glass fermenter | Biogas yield: 265 L/kg biomass; methane concentration: 65% | [94] |
| Jatropha seed cake | Co-digestion of jatropha cake and cattle dung in a 6-m3 floating-type digester for 60 days. | Methane concentration: 62.3–69.2% under mesophilic conditions and 65.2–69.2% for psychrophilic conditions. | [60] |
| Jatropha seed cake | Pilot-scale continuous 40-m3 stirred digester; co-digestion with cow dung (3:1) for 120 days | Within 5 days, the reactor started producing 20 m3 of biogas per day. | [95] |
| Jatropha seed cake | Co-digestion with sugarcane bagasse and addition of Fe2+ ions in 120-mL serum vials as digesters. | Co-digestion of jatropha cake (10% (w/v)) and bagasse (5% (w/v)) gave higher BPR than experiments with jatropha cake alone. Adding 10 mM of Fe2+ ions led to further improvement. | [96] |
| Jatropha seed cake | AD in the presence of an iron additive | H2S content in biogas was reduced. | [97] |
| Jatropha and karanja cakes | AD in a 20 m3/d floating drum under mesophilic temperature | Methane potential: 0.39 (for jatropha cake) and 0.43 m3/kg TS (for karanja cake); average methane concentration: 66.6% (for jatropha) and 62.5% (for karanja); higher methane concentration than in biogas from cattle dung. | [98] |
| Jatropha and karanja cakes, pods, and glycerol | Serum glass bottles (125 mL) fitted with rubber airtight stoppers were used as digesters. | The biogas potential of residues of karanja and jatropha was, respectively, 3.07 and 1.83 m3 per kg of produced biodiesel. | [56] |
| Karanja oil cake | Karanja cake mixed with cow dung in 75:25, 50:50, 25:75 and 0:100 (w/w) proportions | The 25:75 mixture gave the best results. Methane content was 73%, and the slurry had a higher fertilizer value. | [99] |
| Mahua and hingan cakes | A 20-L plastic bottle was used as single-phase digestion system | Biogas yield: 198–233 L/kg seedcake. The digestates had high fertilizer value due to high nitrogen content. | [100] |
| Castor cake | AD in 5-L capacity single-stage fermenters at 30 and 37 °C | Particle size 2.0–1.4 mm was favorable for BPR. High temperature resulted in higher yield. Conversion of the feed: 30–35% TS. | [101] |
| Castor cake, stem, and leaves | AD in 118-mL bottles | Seed cakes and leaves were suitable substrates for AD, but stems were unsuitable without pretreatment. The combined biogas yield from cake, stem, and leaves was 131 g/kg of initial plant biomass. Biodiesel yield is 155 g/kg, and ethanol yield is 85 g/kg. | [57] |
4.1.2. Sugar-Platform Processes
4.1.3. Production of Enzymes from Residues of Non-Edible Oilseeds
4.2. Other Valorization Routes for Non-Edible Oilseed Residues
5. Pretreatment of Non-Edible Oilseed Residues for Bioconversion
5.1. Pretreatment for Sugar-Platform Processes
5.2. Pretreatment for Anaerobic Digestion
6. Future Directions and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afonso, T.L.; Marques, A.C.; Fuinhas, J.A. Strategies to Make Renewable Energy Sources Compatible with Economic Growth. Energy Strategy Rev. 2017, 18, 121–126. [Google Scholar] [CrossRef]
- Yangin-Gomec, C.; Sárvári Horváth, I.; Martín, C. Energy Production from Biomass Valorization. Energies 2023, 16, 4300. [Google Scholar] [CrossRef]
- Yang, L.; Wang, X.-C.; Dai, M.; Chen, B.; Qiao, Y.; Deng, H.; Zhang, D.; Zhang, Y.; Villas Bôas de Almeida, C.M.; Chiu, A.S.F.; et al. Shifting from Fossil-Based Economy to Bio-Based Economy: Status Quo, Challenges, and Prospects. Energy 2021, 228, 120533. [Google Scholar] [CrossRef]
- Sun, J.; Peng, H.; Chen, J.; Wang, X.; Wei, M.; Li, W.; Yang, L.; Zhang, Q.; Wang, W.; Mellouki, A. An Estimation of CO2 Emission via Agricultural Crop Residue Open Field Burning in China from 1996 to 2013. J. Clean. Prod. 2016, 112, 2625–2631. [Google Scholar] [CrossRef]
- Rulli, M.C.; Bellomi, D.; Cazzoli, A.; De Carolis, G.; D’Odorico, P. The Water-Land-Food Nexus of First-Generation Biofuels. Sci. Rep. 2016, 6, 22521. [Google Scholar] [CrossRef]
- Stephen, J.D.; Mabee, W.E.; Saddler, J.N. Will Second-Generation Ethanol Be Able to Compete with First-Generation Ethanol? Opportunities for Cost Reduction. Biofuels Bioprod. Biorefining 2012, 6, 159–176. [Google Scholar] [CrossRef]
- Qiu, F.; Li, Y.; Yang, D.; Li, X.; Sun, P. Biodiesel Production from Mixed Soybean Oil and Rapeseed Oil. Appl. Energy 2011, 88, 2050–2055. [Google Scholar] [CrossRef]
- Kant Bhatia, S.; Kant Bhatia, R.; Jeon, J.-M.; Pugazhendhi, A.; Kumar Awasthi, M.; Kumar, D.; Kumar, G.; Yoon, J.-J.; Yang, Y.-H. An Overview on Advancements in Biobased Transesterification Methods for Biodiesel Production: Oil Resources, Extraction, Biocatalysts, and Process Intensification Technologies. Fuel 2021, 285, 119117. [Google Scholar] [CrossRef]
- EIA—U.S. Energy Information Administration. Official Energy Statistics from the U.S. Government. Available online: https://www.eia.gov/index.php (accessed on 19 July 2023).
- Afriyanti, D.; Kroeze, C.; Saad, A. Indonesia Palm Oil Production without Deforestation and Peat Conversion by 2050. Sci. Total Environ. 2016, 557–558, 562–570. [Google Scholar] [CrossRef]
- Vossen, P. Olive Oil: History, Production, and Characteristics of the World’s Classic Oils. HortScience 2007, 42, 1093–1100. [Google Scholar] [CrossRef]
- Fagionato Masiero, J.; Barbosa, E.J.; de Oliveira Macedo, L.; de Souza, A.; Nishitani Yukuyama, M.; Arantes, G.J.; Bou-Chacra, N.A. Vegetable Oils in Pharmaceutical and Cosmetic Lipid-Based Nanocarriers Preparations. Ind. Crops Prod. 2021, 170, 113838. [Google Scholar] [CrossRef]
- Wen, C.; Shen, M.; Liu, G.; Liu, X.; Liang, L.; Li, Y.; Zhang, J.; Xu, X. Edible Vegetable Oils from Oil Crops: Preparation, Refining, Authenticity Identification and Application. Process Biochem. 2023, 124, 168–179. [Google Scholar] [CrossRef]
- Shailaja, P.; Vinitha, B. Production of Biodiesel from Vegetable Oils. Helix-Sci. Explor. 2018, 8, 3394–3398. [Google Scholar] [CrossRef]
- Farobie, O.; Hartulistiyoso, E. Palm Oil Biodiesel as a Renewable Energy Resource in Indonesia: Current Status and Challenges. BioEnergy Res. 2022, 15, 93–111. [Google Scholar] [CrossRef]
- Sharma, A.; Melo, J.S.; Prakash, R.; Tejo Prakash, N. Lab-Scale Production of Biodiesel from Soybean Acid Oil Using Immobilized Whole Cells as Catalyst. Biocatal. Biotransform. 2021, 39, 443–454. [Google Scholar] [CrossRef]
- Charpe, T.W.; Rathod, V.K. Biodiesel Production Using Waste Frying Oil. Waste Manag. 2011, 31, 85–90. [Google Scholar] [CrossRef]
- Shaah, M.A.H.; Sohrab Hossain, M.; Allafi, F.A.S.; Alsaedi, A.; Ismail, N.; Kadir, M.O.A.; Idayu Ahmad, M. A Review on Non-Edible Oil as a Potential Feedstock for Biodiesel: Physicochemical Properties and Production Technologies. RSC Adv. 2021, 11, 25018–25037. [Google Scholar] [CrossRef]
- Martín, C.; Moure, A.; Martín, G.; Carrillo, E.; Domínguez, H.; Parajó, J.C. Fractional Characterisation of Jatropha, Neem, Moringa, Trisperma, Castor and Candlenut Seeds as Potential Feedstocks for Biodiesel Production in Cuba. Biomass Bioenergy 2010, 34, 533–538. [Google Scholar] [CrossRef]
- No, S.-Y. Inedible Vegetable Oils and Their Derivatives for Alternative Diesel Fuels in CI Engines: A Review. Renew. Sustain. Energy Rev. 2011, 15, 131–149. [Google Scholar] [CrossRef]
- Visser, E.M.; Filho, D.O.; Martins, M.A.; Steward, B.L. Bioethanol Production Potential from Brazilian Biodiesel Co-Products. Biomass Bioenergy 2011, 35, 489–494. [Google Scholar] [CrossRef]
- Jingura, R.M.; Kamusoko, R. Technical Options for Valorisation of Jatropha Press-Cake: A Review. Waste Biomass Valor. 2018, 9, 701–713. [Google Scholar] [CrossRef]
- Demirbas, A.; Bafail, A.; Ahmad, W.; Sheikh, M. Biodiesel Production from Non-Edible Plant Oils. Energy Explor. Exploit. 2016, 34, 290–318. [Google Scholar] [CrossRef]
- Gui, M.M.; Lee, K.T.; Bhatia, S. Feasibility of Edible Oil vs. Non-Edible Oil vs. Waste Edible Oil as Biodiesel Feedstock. Energy 2008, 33, 1646–1653. [Google Scholar] [CrossRef]
- Mohanty, A.; Rout, P.R.; Dubey, B.; Meena, S.S.; Pal, P.; Goel, M. A Critical Review on Biogas Production from Edible and Non-Edible Oil Cakes. Biomass Convers. Biorefinery 2022, 12, 949–966. [Google Scholar] [CrossRef] [PubMed]
- Muktham, R.; Ball, A.S.; Bhargava, S.K.; Bankupalli, S. Bioethanol Production from Non-Edible de-Oiled Pongamia Pinnata Seed Residue-Optimization of Acid Hydrolysis Followed by Fermentation. Ind. Crops Prod. 2016, 94, 490–497. [Google Scholar] [CrossRef]
- Radhakumari, M.; Ball, A.; Bhargava, S.K.; Satyavathi, B. Optimization of Glucose Formation in Karanja Biomass Hydrolysis Using Taguchi Robust Method. Bioresour. Technol. 2014, 166, 534–540. [Google Scholar] [CrossRef]
- Mohibbe Azam, M.; Waris, A.; Nahar, N.M. Prospects and Potential of Fatty Acid Methyl Esters of Some Non-Traditional Seed Oils for Use as Biodiesel in India. Biomass Bioenergy 2005, 29, 293–302. [Google Scholar] [CrossRef]
- Ashraful, A.M.; Masjuki, H.H.; Kalam, M.A.; Rizwanul Fattah, I.M.; Imtenan, S.; Shahir, S.A.; Mobarak, H.M. Production and Comparison of Fuel Properties, Engine Performance, and Emission Characteristics of Biodiesel from Various Non-Edible Vegetable Oils: A Review. Energy Convers. Manag. 2014, 80, 202–228. [Google Scholar] [CrossRef]
- Biswal, A.K.; Lenka, C.; Panda, P.K.; Yang, J.-M.; Misra, P.K. Investigation of the Functional and Thermal Properties of Mahua Deoiled Cake Flour and Its Protein Isolate for Prospective Food Applications. LWT 2021, 137, 110459. [Google Scholar] [CrossRef]
- Gandhi, S.S.; Gogate, P.R.; Pakhale, V.D. Intensification of Interesterification of Sustainable Feedstock as Mahua Oil for Biodiesel Production. Int. J. Green Energy 2022, 1–10. [Google Scholar] [CrossRef]
- Aransiola, E.F.; Betiku, E.; Ikhuomoregbe, D.I.O.; Ojumu, T.V. Production of Biodiesel from Crude Neem Oil Feedstock and Its Emissions from Internal Combustion Engines. Afr. J. Biotechnol. 2012, 11, 6178–6186. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Ong, H.C.; Mahlia, T.M.I.; Masjuki, H.H.; Badruddin, I.A.; Fayaz, H. Non-Edible Vegetable Oils: A Critical Evaluation of Oil Extraction, Fatty Acid Compositions, Biodiesel Production, Characteristics, Engine Performance and Emissions Production. Renew. Sustain. Energy Rev. 2013, 18, 211–245. [Google Scholar] [CrossRef]
- Park, J.-Y.; Kim, D.-K.; Wang, Z.-M.; Lu, P.; Park, S.-C.; Lee, J.-S. Production and Characterization of Biodiesel from Tung Oil. Appl. Biochem. Biotechnol. 2008, 148, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Jiang, W.; Lu, H.; Liang, B. Properties of Tung Oil Biodiesel and Its Blends with 0# Diesel. Bioresour. Technol. 2010, 101, 826–828. [Google Scholar] [CrossRef] [PubMed]
- Omonhinmin, C.; Olomukoro, E.; Ayoola, A.; Egwim, E.; Omonhinmin, C.; Olomukoro, E.; Ayoola, A.; Egwim, E. Utilization of Moringa oleifera Oil for Biodiesel Production: A Systematic Review. AIMSE 2020, 8, 102–121. [Google Scholar] [CrossRef]
- Norazahar, N.; Yusup, S.; Ahmad, M.M.; Bakar, S.A.; Ahmad, J. Parametric Optimization of Kapok (Ceiba pentandra) Oil Methyl Ester Production Using Taguchi Approach. Int. J. Energy Environ. 2012, 6, 541–548. [Google Scholar]
- Dharma, S.; Masjuki, H.H.; Ong, H.C.; Sebayang, A.H.; Silitonga, A.S.; Kusumo, F.; Mahlia, T.M.I. Optimization of Biodiesel Production Process for Mixed Jatropha curcas–Ceiba pentandra Biodiesel Using Response Surface Methodology. Energy Convers. Manag. 2016, 115, 178–190. [Google Scholar] [CrossRef]
- Sánchez, M.; Avhad, M.R.; Marchetti, J.M.; Martínez, M.; Aracil, J. Jojoba Oil: A State of the Art Review and Future Prospects. Energy Convers. Manag. 2016, 129, 293–304. [Google Scholar] [CrossRef]
- Gowtham Rajan, A.; Sivasubramanian, M.; Gowthaman, S.; Ramkumar, P. Investigation of Physical and Chemical Properties of Tobacco Seed Oil Fatty Acid Methyl Ester for Biodiesel Production. Mater. Today Proc. 2021, 46, 7670–7675. [Google Scholar] [CrossRef]
- Banković-Ilić, I.B.; Stamenković, O.S.; Veljković, V.B. Biodiesel Production from Non-Edible Plant Oils. Renew. Sustain. Energy Rev. 2012, 16, 3621–3647. [Google Scholar] [CrossRef]
- Wu, S.; Gao, C.; Pan, H.; Wei, K.; Li, D.; Cai, K.; Zhang, H. Advancements in Tobacco (Nicotiana tabacum L.) Seed Oils for Biodiesel Production. Front. Chem. 2022, 9, 834936. [Google Scholar] [CrossRef]
- Dawood, S.; Ahmad, M.; Ullah, K.; Zafar, M.; Khan, K. Synthesis and Characterization of Methyl Esters from Non-Edible Plant Species Yellow Oleander Oil, Using Magnesium Oxide (MgO) Nano-Catalyst. Mater. Res. Bull. 2018, 101, 371–379. [Google Scholar] [CrossRef]
- Deshmukh, S.J.; Bhuyar, L.B. Transesterified Hingan (Balanites) Oil as a Fuel for Compression Ignition Engines. Biomass Bioenergy 2009, 33, 108–112. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Mahlia, T.M.I.; Ong, H.C.; Riayatsyah, T.M.I.; Kusumo, F.; Ibrahim, H.; Dharma, S.; Gumilang, D. A Comparative Study of Biodiesel Production Methods for Reutealis trisperma Biodiesel. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 2006–2014. [Google Scholar] [CrossRef]
- Kansedo, J.; Lee, K.T. Process Optimization and Kinetic Study for Biodiesel Production from Non-Edible Sea Mango (Cerbera odollam) Oil Using Response Surface Methodology. Chem. Eng. J. 2013, 214, 157–164. [Google Scholar] [CrossRef]
- Shaah, M.A.; Allafi, F.; Hossain, M.S.; Alsaedi, A.; Ismail, N.; Kadir, M.O.A.; Ahmad, M.I. Candlenut Oil: Review on Oil Properties and Future Liquid Biofuel Prospects. Int. J. Energy Res. 2021, 45, 17057–17079. [Google Scholar] [CrossRef]
- Dierig, D.A.; Wang, G.; McCloskey, W.B.; Thorp, K.R.; Isbell, T.A.; Ray, D.T.; Foster, M.A. Lesquerella: New Crop Development and Commercialization in the U.S. Ind. Crops Prod. 2011, 34, 1381–1385. [Google Scholar] [CrossRef]
- Dawood, S.; Koyande, A.K.; Ahmad, M.; Mubashir, M.; Asif, S.; Klemeš, J.J.; Bokhari, A.; Saqib, S.; Lee, M.; Qyyum, M.A.; et al. Synthesis of Biodiesel from Non-Edible (Brachychiton populneus) Oil in the Presence of Nickel Oxide Nanocatalyst: Parametric and Optimisation Studies. Chemosphere 2021, 278, 130469. [Google Scholar] [CrossRef]
- Prabhu, A.; Venkata Ramanan, M.; Jayaprabakar, J. Production, Properties and Engine Characteristics of Jatropha Biodiesel—A Review. Int. J. Ambient. Energy 2021, 42, 1810–1814. [Google Scholar] [CrossRef]
- Gübitz, G.M.; Mittelbach, M.; Trabi, M. Exploitation of the Tropical Oil Seed Plant Jatropha curcas L. Bioresour. Technol. 1999, 67, 73–82. [Google Scholar] [CrossRef]
- Ewunie, G.A.; Morken, J.; Lekang, O.I.; Yigezu, Z.D. Factors Affecting the Potential of Jatropha curcas for Sustainable Biodiesel Production: A Critical Review. Renew. Sustain. Energy Rev. 2021, 137, 110500. [Google Scholar] [CrossRef]
- Singh, R.N.; Vyas, D.K.; Srivastava, N.S.L.; Narra, M. SPRERI Experience on Holistic Approach to Utilize All Parts of Jatropha curcas Fruit for Energy. Renew. Energy 2008, 33, 1868–1873. [Google Scholar] [CrossRef]
- García, A.; López, Y.; Karimi, K.; Benítez, A.; Lundin, M.; Taherzadeh, M.; Martín, C. Chemical and Physical Characterization and Acid Hydrolysis of a Mixture of Jatropha curcas Shells and Husks. Cell. Chem. Technol. 2015, 49, 737–744. [Google Scholar]
- Liang, Y.; Siddaramu, T.; Yesuf, J.; Sarkany, N. Fermentable Sugar Release from Jatropha Seed Cakes Following Lime Pretreatment and Enzymatic Hydrolysis. Bioresour. Technol. 2010, 101, 6417–6424. [Google Scholar] [CrossRef] [PubMed]
- Khuntia, H.K.; Chanakya, H.N.; Siddiqha, A.; Thomas, C.; Mukherjee, N.; Janardhana, N. Anaerobic Digestion of the Inedible Oil Biodiesel Residues for Value Addition. Sustain. Energy Technol. Assess. 2017, 22, 9–17. [Google Scholar] [CrossRef]
- Bateni, H.; Karimi, K.; Zamani, A.; Benakashani, F. Castor Plant for Biodiesel, Biogas, and Ethanol Production with a Biorefinery Processing Perspective. Appl. Energy 2014, 136, 14–22. [Google Scholar] [CrossRef]
- Abdelmoez, W.; Tayeb, A.M.; Mustafa, A.; Abdelhamid, M. Green Approach for Biodiesel Production from Jojoba Oil Supported by Process Modeling and Simulation. Int. J. Chem. React. Eng. 2016, 14, 185–193. [Google Scholar] [CrossRef]
- Arrutia, F.; Binner, E.; Williams, P.; Waldron, K.W. Oilseeds beyond Oil: Press Cakes and Meals Supplying Global Protein Requirements. Trends Food Sci. Technol. 2020, 100, 88–102. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sahoo, P.K.; Mukherjee, M.; Patel, A. Assessment of Sustainable Biogas Production from Co-Digestion of Jatropha De-Oiled Cake and Cattle Dung Using Floating Drum Type Digester under Psychrophilic and Mesophilic Conditions. Clean. Technol. 2022, 4, 529–541. [Google Scholar] [CrossRef]
- Oruganti, R.K.; Gungupalli, M.P.; Bhattacharyya, D. Alkaline Hydrolysis for Yield of Glucose and Kraft Lignin from De-Oiled Jatropha curcas Waste: Multiresponse Optimization Using Response Surface Methodology. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Al-Zoubi, H.; Zubair, M.; Manzar, M.S.; Manda, A.A.; Blaisi, N.I.; Qureshi, A.; Matani, A. Comparative Adsorption of Anionic Dyes (Eriochrome Black T and Congo Red) onto Jojoba Residues: Isotherm, Kinetics and Thermodynamic Studies. Arab. J. Sci. Eng. 2020, 45, 7275–7287. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, A.; Sharma, S.; Vijay, V.K. Comparative Evaluation of Raw and Detoxified Mahua Seed Cake for Biogas Production. Appl. Energy 2013, 102, 1514–1521. [Google Scholar] [CrossRef]
- Doshi, P.; Srivastava, G. Sustainable Approach to Produce Bioethanol from Karanja (Pongamia pinnata) Oilseed Residue. Turk. J. Agric. 2013, 37, 781–788. [Google Scholar] [CrossRef]
- Madeira, J.V.; Macedo, J.A.; Macedo, G.A. Detoxification of Castor Bean Residues and the Simultaneous Production of Tannase and Phytase by Solid-State Fermentation Using Paecilomyces variotii. Bioresour. Technol. 2011, 102, 7343–7348. [Google Scholar] [CrossRef]
- Sharma, R.; Sheth, P.N.; Gujrathi, A.M. Kinetic Modeling and Simulation: Pyrolysis of Jatropha Residue de-Oiled Cake. Renew. Energy 2016, 86, 554–562. [Google Scholar] [CrossRef]
- Pari, L.; Suardi, A.; Longo, L.; Carnevale, M.; Gallucci, F. Jatropha curcas L. Pruning Residues for Energy: Characteristics of an Untapped By-Product. Energies 2018, 11, 1622. [Google Scholar] [CrossRef]
- Kaur, R.; Gera, P.; Jha, M.K.; Bhaskar, T. Hydrothermal Treatment of Pretreated Castor Residue for the Production of Bio-Oil. BioEnergy Res. 2023, 16, 517–527. [Google Scholar] [CrossRef]
- Grigoriou, A.H.; Ntalos, G.A. The Potential Use of Ricinus communis L. (Castor) Stalks as a Lignocellulosic Resource for Particleboards. Ind. Crops Prod. 2001, 13, 209–218. [Google Scholar] [CrossRef]
- Saleh, A.A.; Paray, B.A.; Dawood, M.A.O. Olive Cake Meal and Bacillus licheniformis Impacted the Growth Performance, Muscle Fatty Acid Content, and Health Status of Broiler Chickens. Animals 2020, 10, 695. [Google Scholar] [CrossRef]
- Cordeiro, C.N.; Freitas, E.R.; Nepomuceno, R.C.; Pinheiro, S.G.; Souza, D.H.; Watanabe, G.C.A.; Freitas, C.A.; Watanabe, P.H. Nutritional Composition, Metabolisable Energy and Total Use of Sunflower Seed Cake for Meat Quail. Braz. J. Poult. Sci. 2022, 24, eRBCA. [Google Scholar] [CrossRef]
- Rakita, S.; Kokić, B.; Manoni, M.; Mazzoleni, S.; Lin, P.; Luciano, A.; Ottoboni, M.; Cheli, F.; Pinotti, L. Cold-Pressed Oilseed Cakes as Alternative and Sustainable Feed Ingredients: A Review. Foods 2023, 12, 432. [Google Scholar] [CrossRef] [PubMed]
- Sunil, L.; Appaiah, P.; Prasanth Kumar, P.K.; Gopala Krishna, A.G. Preparation of Food Supplements from Oilseed Cakes. J. Food Sci. Technol. 2015, 52, 2998–3005. [Google Scholar] [CrossRef] [PubMed]
- Azari, S.R.; Hojjatoleslamy, M.; Mousavi, Z.E.; Kiani, H.; Jalali, S.M.A. Production and Optimization of Conjugated Linoleic and Eicosapentaenoic Acids by Bifidobacterium lactis in Cold-Pressed Soybean Cake. Front. Nutr. 2022, 9, 916728. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Singh, S.K.; Larroche, C.; Soccol, C.R.; Pandey, A. Oil Cakes and Their Biotechnological Applications—A Review. Bioresour. Technol. 2007, 98, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Kethobile, E.; Ketlogetswe, C.; Gandure, J. Characterisation of the Non-Oil Jatropha Biomass Material for Use as a Source of Solid Fuel. Biomass Convers. Biorefinery 2020, 10, 1251–1267. [Google Scholar] [CrossRef]
- Arulprakasajothi, M.; Beemkumar, N.; Parthipan, J.; Battu, N. raju Investigating the Physio-Chemical Properties of Densified Biomass Pellet Fuels from Fruit and Vegetable Market Waste. Arab. J. Sci. Eng. 2020, 45, 563–574. [Google Scholar] [CrossRef]
- Al-Widyan, M.I.; Al-Muhtaseb, M.A. Experimental Investigation of Jojoba as a Renewable Energy Source. Energy Convers. Manag. 2010, 51, 1702–1707. [Google Scholar] [CrossRef]
- Luo, M.; Wang, S.; Wang, L.; Lv, M.; Qian, L.; Fu, H. Experimental Investigation of Co-Combustion of Coal and Biomass Using Chemical Looping Technology. Fuel Process. Technol. 2013, 110, 258–267. [Google Scholar] [CrossRef]
- Eswanto; Siahaan, J.R. Analysis of Castel Type Biomass Combustion Chamber Using Candlenut Shell Fuel for Patchouli Oil Purifying. J. Mech. Eng. Sci. 2018, 12, 3656–3670. [Google Scholar] [CrossRef]
- Pasciucco, F.; Francini, G.; Pecorini, I.; Baccioli, A.; Lombardi, L.; Ferrari, L. Valorization of Biogas from the Anaerobic Co-Treatment of Sewage Sludge and Organic Waste: Life Cycle Assessment and Life Cycle Costing of Different Recovery Strategies. J. Clean. Prod. 2023, 401, 136762. [Google Scholar] [CrossRef]
- Pecorini, I.; Peruzzi, E.; Albini, E.; Doni, S.; Macci, C.; Masciandaro, G.; Iannelli, R. Evaluation of MSW Compost and Digestate Mixtures for a Circular Economy Application. Sustainability 2020, 12, 3042. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of Lignocellulose: Formation of Inhibitory by-Products and Strategies for Minimizing Their Effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Gandla, M.L.; Tang, C.; Jönsson, L.J.; Martín, C. Enzymatic Saccharification of Lignocellulosic Biomass. In Enzymes in Agriculture and Industry; Agricultural Biocatalysis; Jenny Stanford Publishing: Singapore, 2022; Volume 9, pp. 413–469. [Google Scholar]
- Chacón-Navarrete, H.; Martín, C.; Moreno-García, J. Yeast Immobilization Systems for Second-Generation Ethanol Production: Actual Trends and Future Perspectives. Biofuels Bioprod. Biorefining 2021, 15, 1549–1565. [Google Scholar] [CrossRef]
- dos Santos, J.R.A.; Souto-Maior, A.M.; Gouveia, E.R.; Martín, C. Comparison of SHF and SSF processes from sugar cane bagasse for ethanol production by Saccharomyces cerevisiae|Comparação entre processos em SHF e em SSF de bagaço de cana-de-açúcar para a produção de etanol por Saccharomyces cerevisiae. Quím. Nova 2010, 33, 904–908. [Google Scholar] [CrossRef]
- Olguin-Maciel, E.; Singh, A.; Chable-Villacis, R.; Tapia-Tussell, R.; Ruiz, H.A. Consolidated Bioprocessing, an Innovative Strategy towards Sustainability for Biofuels Production from Crop Residues: An Overview. Agronomy 2020, 10, 1834. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, A.; Pathak, R.; Kumar, A.; Sharma, S. Solid State Fermentation of Non-Edible Oil Seed Cakes for Production of Proteases and Cellulases and Degradation of AntiNutritional Factors. J. Food Biotechnol. Res. 2018, 2, 1:4. [Google Scholar]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-Industrial Wastes and Their Utilization Using Solid State Fermentation: A Review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Staubmann, R.; Foidl, G.; Foidl, N.; Gübitz, G.M.; Lafferty, R.M.; Arbizu, V.M.; Steiner, W. Biogas Production from Jatropha curcas Press-Cake. Appl. Biochem. Biotechnol. 1997, 63–65, 457–467. [Google Scholar] [CrossRef]
- Sinbuathong, N.; Munakata-Marr, J.; Sillapacharoenkul, B.; Chulalaksananukul, S. Effect of the Solid Content on Biogas Production from Jatropha curcas Seed Cake. Int. J. Glob. Warm. 2011, 3, 403–416. [Google Scholar] [CrossRef]
- Sinbuathong, N.; Sillapacharoenkul, B.; Khun-Anake, R.; Watts, D. Optimum Organic Loading Rate for Semi-Continuous Operation of an Anaerobic Process for Biogas Production from Jatropha curcas Seed Cake. Int. J. Glob. Warm. 2010, 2, 179–188. [Google Scholar] [CrossRef]
- Raheman, H.; Mondal, S. Biogas Production Potential of Jatropha Seed Cake. Biomass Bioenergy 2012, 37, 25–30. [Google Scholar] [CrossRef]
- Chandra, R.; Vijay, V.; Subbarao, P. A Study on Biogas Generation from Non-Edible Oil Seed Cakes: Potential and Prospects in India. In Proceedings of the 2nd Joint International Conference on Sustainable Energy and Environment, Bangkok, Thailand, 21–23 November 2006. [Google Scholar]
- Singhal, S.; Agarwal, S.; Singhal, N.; Sharma, R.; Sharma, R. Designing and Operation of Pilot Scale Continuous Stirred Tank Reactor for Continuous Production of Bio-Methane from Toxic Waste. Environ. Prog. Sustain. Energy 2019, 38, 198–200. [Google Scholar] [CrossRef]
- Sen, K.; Mahalingam, S.; Sen, B. Rapid and High Yield Biogas Production from Jatropha Seed Cake by Co-Digestion with Bagasse and Addition of Fe2+. Environ. Technol. 2013, 34, 2989–2994. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T. Anaerobic Digestion of Jatropha curcas L. Press Cake and Effects of an Iron-Additive. Waste Manag. Res. 2011, 29, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Vijay, V.K.; Subbarao, P.M.V.; Khura, T.K. Production of Methane from Anaerobic Digestion of Jatropha and Pongamia Oil Cakes. Appl. Energy 2012, 93, 148–159. [Google Scholar] [CrossRef]
- Barik, D.; Murugan, S. Assessment of Sustainable Biogas Production from De-Oiled Seed Cake of Karanja-an Organic Industrial Waste from Biodiesel Industries. Fuel 2015, 148, 25–31. [Google Scholar] [CrossRef]
- Deshpande, N.V.; Kale, N.W.; Deshmukh, S.J. A Study on Biogas Generation from Mahua (Madhuca indica) and Hingan (Balanites aegyaptiaca) Oil Seedcake. Energy Sustain. Dev. 2012, 16, 363–367. [Google Scholar] [CrossRef]
- Gollakota, K.G.; Meher, K.K. Effect of Particle Size, Temperature, Loading Rate and Stirring on Biogas Production from Castor Cake (Oil Expelled). Biol. Wastes 1988, 24, 243–249. [Google Scholar] [CrossRef]
- Muhammad, M.B.; Chandra, R. Enhancing Biogas and Methane Production from Leaf Litter of Neem by Co-Digestion with Vegetable Waste: Focus on the Effect of Tannin. Biomass Bioenergy 2021, 147, 106007. [Google Scholar] [CrossRef]
- Tambone, F.; Pradella, M.; Bedussi, F.; Adani, F. Moringa oleifera Lam. as an Energy Crop for Biogas Production in Developing Countries. Biomass Convers. Biorefinery 2020, 10, 1083–1089. [Google Scholar] [CrossRef]
- Gaul, M. An Analysis Model for Small-Scale Rural Energy Service Pathway—Applied to Jatropha-Based Energy Services in Sumbawa, Indonesia. Energy Sustain. Dev. 2012, 16, 283–296. [Google Scholar] [CrossRef]
- Palit, D.; Malhotra, R.; Mande, S. Enhancing Viability of Biofuel-Based Decentralized Power Projects for Rural Electrification in India. Environ. Dev. Sustain. 2017, 19, 263–283. [Google Scholar] [CrossRef]
- Momayez, F.; Hedenström, M.; Stagge, S.; Jönsson, L.J.; Martín, C. Valorization of Hydrolysis Lignin from a Spruce-Based Biorefinery by Applying γ-Valerolactone Treatment. Bioresour. Technol. 2022, 359, 127466. [Google Scholar] [CrossRef] [PubMed]
- Martín, C.; García, A.; Schreiber, A.; Puls, J.; Saake, B. Combination of Water Extraction with Dilute-Sulphuric Acid Pretreatment for Enhancing the Enzymatic Hydrolysis of Jatropha curcas Shells. Ind. Crops Prod. 2015, 64, 233–241. [Google Scholar] [CrossRef]
- Marasabessy, A.; Kootstra, A.M.J.; Sanders, J.P.; Weusthuis, R.A. Dilute H2SO4-Catalyzed Hydrothermal Pretreatment to Enhance Enzymatic Digestibility of Jatropha curcas Fruit Hull for Ethanol Fermentation. Int. J. Energy Environ. Eng. 2012, 3, 15. [Google Scholar] [CrossRef]
- Kumar, G.; Sen, B.; Lin, C.-Y. Pretreatment and Hydrolysis Methods for Recovery of Fermentable Sugars from De-Oiled Jatropha Waste. Bioresour. Technol. 2013, 145, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Sen, B.; Sivagurunathan, P.; Lin, C.-Y. High Rate Hydrogen Fermentation of Cello-Lignin Fraction in de-Oiled Jatropha Waste Using Hybrid Immobilized Cell System. Fuel 2016, 182, 131–140. [Google Scholar] [CrossRef]
- Garza, J.a.R.L.; Castillo-Quiroz, D.; Ríos-González, L.; Morales-Martínez, T.; González-Fuentes, J.A.; Valdez-Aguilar, L.; Medina-Morales, M.A. Autohydrolysis Pretreatment of Castor Plant Pruning Residues to Enhance Enzymatic Digestibility and Bioethanol Production. Bioresources 2020, 15, 6206–6216. [Google Scholar] [CrossRef]
- Abada, E.; Al-Fifi, Z.; Osman, M. Bioethanol Production with Carboxymethylcellulase of Pseudomonas poae Using Castor Bean (Ricinus communis L.) Cake. Saudi J. Biol. Sci. 2019, 26, 866–871. [Google Scholar] [CrossRef]
- Radhakumari, M.; Taha, M.; Shahsavari, E.; Bhargava, S.K.; Satyavathi, B.; Ball, A.S. Pongamia pinnata Seed Residue—A Low Cost Inedible Resource for on-Site/in-House Lignocellulases and Sustainable Ethanol Production. Renew. Energy 2017, 103, 682–687. [Google Scholar] [CrossRef]
- Hernández, E.; García, A.; López, M.; Puls, J.; Parajó, J.C.; Martín, C. Dilute Sulphuric Acid Pretreatment and Enzymatic Hydrolysis of Moringa oleifera Empty Pods. Ind. Crops Prod. 2013, 44, 227–231. [Google Scholar] [CrossRef]
- Montaño, H.F.; Rincón, S.L.; Serrato, J.C. Study of the Influence of Dilute Acid Pre-Treatment Conditions on Glucose Recovery from Jatropha curcas Lam for Fuel-Ethanol Production. Int. J. Green Energy 2017, 14, 613–623. [Google Scholar] [CrossRef]
- Harry-O’kuru, R.E.; Gordon, S.H.; Klokkenga, M. Bio-Generation of Succinic Acid by Fermentation of Physaria fendleri Seed Polysaccharides. Ind. Crops Prod. 2015, 77, 116–122. [Google Scholar] [CrossRef]
- Kumar, G.; Sivagurunathan, P.; Lin, C.-Y. Influence of Various Combinations of Heat Pretreatment on Hydrogen Fermentation from Deoiled Jatropha Waste Using Microflora. Environ. Eng. Manag. J. 2019, 18, 1–7. [Google Scholar] [CrossRef]
- Qureshi, N.; Harry-O’kuru, R.; Liu, S.; Saha, B. Yellow Top (Physaria fendleri) Presscake: A Novel Substrate for Butanol Production and Reduction in Environmental Pollution. Biotechnol. Prog. 2019, 35, e2767. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.-C.; Hsu, H.-Y.; Jang, G.-W. Biological Routes to Itaconic and Succinic Acids. Phys. Sci. Rev. 2016, 1, 20160052. [Google Scholar] [CrossRef]
- Jiang, L.; Fang, Z.; Li, X.-K.; Luo, J. Production of 2,3-Butanediol from Cellulose and Jatropha Hulls after Ionic Liquid Pretreatment and Dilute-Acid Hydrolysis. AMB Express 2013, 3, 48. [Google Scholar] [CrossRef]
- Ancuța, P.; Sonia, A. Oil Press-Cakes and Meals Valorization through Circular Economy Approaches: A Review. Appl. Sci. 2020, 10, 7432. [Google Scholar] [CrossRef]
- Ilmi, M.; Hidayat, C.; Hastuti, P.; Heeres, H.J.; van der Maarel, M.J.E.C. Utilisation of Jatropha Press Cake as Substrate in Biomass and Lipase Production from Aspergillus niger 65I6 and Rhizomucor miehei CBS 360.62. Biocatal. Agric. Biotechnol. 2017, 9, 103–107. [Google Scholar] [CrossRef]
- El-Ghonemy, D.H. Optimization of Extracellular Ethanol-Tolerant β-Glucosidase Production from a Newly Isolated Aspergillus sp. DHE7 via Solid State Fermentation Using Jojoba Meal as Substrate: Purification and Biochemical Characterization for Biofuel Preparation. J. Genet. Eng. Biotechnol. 2021, 19, 45. [Google Scholar] [CrossRef]
- Quiroz-Castañeda, R.E.; Pérez-Mejía, N.; Martínez-Anaya, C.; Acosta-Urdapilleta, L.; Folch-Mallol, J. Evaluation of Different Lignocellulosic Substrates for the Production of Cellulases and Xylanases by the Basidiomycete Fungi Bjerkandera adusta and Pycnoporus sanguineus. Biodegradation 2011, 22, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Montoya, E.L.; Castro-Ochoa, L.D.; Maldonado-Mendoza, I.E.; Luna-Suárez, S.; Castro-Martínez, C. Moringa Straw as Cellulase Production Inducer and Cellulolytic Fungi Source. Rev. Argent. Microbiol. 2020, 52, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Mahanta, N.; Gupta, A.; Khare, S.K. Production of Protease and Lipase by Solvent Tolerant Pseudomonas aeruginosa PseA in Solid-State Fermentation Using Jatropha curcas Seed Cake as Substrate. Bioresour. Technol. 2008, 99, 1729–1735. [Google Scholar] [CrossRef]
- Joshi, C.; Khare, S.K. Utilization of Deoiled Jatropha curcas Seed Cake for Production of Xylanase from Thermophilic Scytalidium thermophilum. Bioresour. Technol. 2011, 102, 1722–1726. [Google Scholar] [CrossRef]
- Dave, B.R.; Sudhir, A.P.; Pansuriya, M.; Raykundaliya, D.P.; Subramanian, R.B. Utilization of Jatropha Deoiled Seed Cake for Production of Cellulases under Solid-State Fermentation. Bioprocess. Biosyst. Eng. 2012, 35, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Ncube, T.; Howard, R.L.; Abotsi, E.K.; van Rensburg, E.L.J.; Ncube, I. Jatropha Curcas Seed Cake as Substrate for Production of Xylanase and Cellulase by Aspergillus niger FGSCA733 in Solid-State Fermentation. Ind. Crops Prod. 2012, 37, 118–123. [Google Scholar] [CrossRef]
- Godoy, M.G.; Gutarra, M.L.E.; Maciel, F.M.; Felix, S.P.; Bevilaqua, J.V.; Machado, O.L.T.; Freire, D.M.G. Use of a Low-Cost Methodology for Biodetoxification of Castor Bean Waste and Lipase Production. Enzym. Microb. Technol. 2009, 44, 317–322. [Google Scholar] [CrossRef]
- Godoy, M.G.; Gutarra, M.L.E.; Castro, A.M.; Machado, O.L.T.; Freire, D.M.G. Adding Value to a Toxic Residue from the Biodiesel Industry: Production of Two Distinct Pool of Lipases from Penicillium simplicissimum in Castor Bean Waste. J. Ind. Microbiol. Biotechnol. 2011, 38, 945–953. [Google Scholar] [CrossRef]
- Herculano, P.N.; Lima, D.M.M.; Fernandes, M.J.S.; Neves, R.P.; Souza-Motta, C.M.; Porto, A.L.F. Isolation of Cellulolytic Fungi from Waste of Castor (Ricinus communis L.). Curr. Microbiol. 2011, 62, 1416–1422. [Google Scholar] [CrossRef]
- Seshagiri, S.; Parthiban, B.; Reddy, N. Production, Properties and Applications of Proteases from Pongamia Oil Seed Cakes. J. Appl. Polym. Sci. 2023, 140, e54280. [Google Scholar] [CrossRef]
- Leite, P.; Sousa, D.; Fernandes, H.; Ferreira, M.; Costa, A.R.; Filipe, D.; Gonçalves, M.; Peres, H.; Belo, I.; Salgado, J.M. Recent Advances in Production of Lignocellulolytic Enzymes by Solid-State Fermentation of Agro-Industrial Wastes. Curr. Opin. Green Sustain. Chem. 2021, 27, 100407. [Google Scholar] [CrossRef]
- Pfeil, M.; Tobío-Pérez, I.; Denfeld, D.; Díaz, Y.; Pohl, S.; Piloto-Rodríguez, R. Characterization and Assessment of Jatropha curcas and Moringa oleifera Husk and Their Potential Use in Gasification. Energ. Ecol. Environ. 2021, 6, 170–182. [Google Scholar] [CrossRef]
- Parascanu, M.M.; Sandoval-Salas, F.; Soreanu, G.; Valverde, J.L.; Sanchez-Silva, L. Valorization of Mexican Biomasses through Pyrolysis, Combustion and Gasification Processes. Renew. Sustain. Energy Rev. 2017, 71, 509–522. [Google Scholar] [CrossRef]
- Majhi, A.; Sharma, Y.K.; Naik, D.V.; Chauhan, R. The Production and Evaluation of Bio-Oil Obtained from the Jatropha curcas Cake. Energy Sources Part A Recovery Util. Environ. Eff. 2015, 37, 1782–1789. [Google Scholar] [CrossRef]
- Sun, K.; Jiang, J. chun Preparation and Characterization of Activated Carbon from Rubber-Seed Shell by Physical Activation with Steam. Biomass Bioenergy 2010, 34, 539–544. [Google Scholar] [CrossRef]
- Onorevoli, B.; da Silva Maciel, G.P.; Machado, M.E.; Corbelini, V.; Caramão, E.B.; Jacques, R.A. Characterization of Feedstock and Biochar from Energetic Tobacco Seed Waste Pyrolysis and Potential Application of Biochar as an Adsorbent. J. Environ. Chem. Eng. 2018, 6, 1279–1287. [Google Scholar] [CrossRef]
- Sowmya Dhanalakshmi, C.; Madhu, P. Biofuel Production of Neem Wood Bark (Azadirachta indica) through Flash Pyrolysis in a Fluidized Bed Reactor and Its Chromatographic Characterization. Energy Sources Part A Recovery Util. Environ. Eff. 2021, 43, 428–443. [Google Scholar] [CrossRef]
- Vinayaka, D.L.; Guna, V.; Madhavi, D.; Arpitha, M.; Reddy, N. Ricinus Communis Plant Residues as a Source for Natural Cellulose Fibers Potentially Exploitable in Polymer Composites. Ind. Crops Prod. 2017, 100, 126–131. [Google Scholar] [CrossRef]
- Kumode, M.M.N.; Bolzon, G.I.M.; Magalhães, W.L.E.; Kestur, S.G. Microfibrillated Nanocellulose from Balsa Tree as Potential Reinforcement in the Preparation of ‘Green’ Composites with Castor Seed Cake. J. Clean. Prod. 2017, 149, 1157–1163. [Google Scholar] [CrossRef]
- Rahman, M.M.; Netravali, A.N. Micro-Fibrillated Cellulose Reinforced Eco-Friendly Polymeric Resin from Non-Edible ‘Jatropha curcas’ Seed Waste after Biodiesel Production. RSC Adv. 2016, 6, 47101–47111. [Google Scholar] [CrossRef]
- Patil, N.V.; Rahman, M.M.; Netravali, A.N. “Green” Composites Using Bioresins from Agro-Wastes and Modified Sisal Fibers. Polym. Compos. 2019, 40, 99–108. [Google Scholar] [CrossRef]
- Rantheesh, J.; Indran, S.; Raja, S.; Siengchin, S. Isolation and Characterization of Novel Micro Cellulose from Azadirachta indica A. Juss Agro-Industrial Residual Waste Oil Cake for Futuristic Applications. Biomass Convers. Biorefinery 2023, 13, 4393–4411. [Google Scholar] [CrossRef]
- Sajithkumar, K.J.; Visakh, P.M.; Ramasamy, E.V. Moringa Oleifera (Drum Stick Vegetable Fibre) Based Nanocomposites with Natural Rubber: Preparation and Characterizations. Waste Biomass Valor. 2016, 7, 1227–1234. [Google Scholar] [CrossRef]
- Evon, P.; Kartika, I.A.; Rigal, L. New Renewable and Biodegradable Particleboards from Jatropha Press Cakes. J. Renew. Mater. 2014, 2, 52–65. [Google Scholar] [CrossRef]
- Liu, C.; Wang, S.; Wang, B.; Song, G. Catalytic Hydrogenolysis of Castor Seeds C-Lignin in Deep Eutectic Solvents. Ind. Crops Prod. 2021, 169, 113666. [Google Scholar] [CrossRef]
- Li, Y.; Shuai, L.; Kim, H.; Motagamwala, A.H.; Mobley, J.K.; Yue, F.; Tobimatsu, Y.; Havkin-Frenkel, D.; Chen, F.; Dixon, R.A.; et al. An “Ideal Lignin” Facilitates Full Biomass Utilization. Sci. Adv. 2018, 4, eaau2968. [Google Scholar] [CrossRef]
- Kandasamy, G.; Shaleh, S.R.M. Flotation Removal of the Microalga Nannochloropsis Sp. Using Moringa Protein–Oil Emulsion: A Novel Green Approach. Bioresour. Technol. 2018, 247, 327–331. [Google Scholar] [CrossRef]
- Feki, F.; Klisurova, D.; Masmoudi, M.A.; Choura, S.; Denev, P.; Trendafilova, A.; Chamkha, M.; Sayadi, S. Optimization of Microwave Assisted Extraction of Simmondsins and Polyphenols from Jojoba (Simmondsia chinensis) Seed Cake Using Box-Behnken Statistical Design. Food Chem. 2021, 356, 129670. [Google Scholar] [CrossRef]
- Oskoueian, E.; Abdullah, N.; Ahmad, S.; Saad, W.Z.; Omar, A.R.; Ho, Y.W. Bioactive Compounds and Biological Activities of Jatropha curcas L. Kernel Meal Extract. Int. J. Mol. Sci. 2011, 12, 5955–5970. [Google Scholar] [CrossRef]
- Galbe, M.; Zacchi, G. Pretreatment of Lignocellulosic Materials for Efficient Bioethanol Production. In Biofuels; Advances in Biochemical Engineering/Biotechnology; Olsson, L., Ed.; Springer: Berlin, Heidelberg, 2007; pp. 41–65. ISBN 978-3-540-73651-6. [Google Scholar]
- Galbe, M.; Wallberg, O. Pretreatment for Biorefineries: A Review of Common Methods for Efficient Utilisation of Lignocellulosic Materials. Biotechnol. Biofuels 2019, 12, 294. [Google Scholar] [CrossRef]
- Kumar, B.; Bhardwaj, N.; Agrawal, K.; Chaturvedi, V.; Verma, P. Current Perspective on Pretreatment Technologies Using Lignocellulosic Biomass: An Emerging Biorefinery Concept. Fuel Process. Technol. 2020, 199, 106244. [Google Scholar] [CrossRef]
- Martín, C.; Dixit, P.; Momayez, F.; Jönsson, L.J. Hydrothermal Pretreatment of Lignocellulosic Feedstocks to Facilitate Biochemical Conversion. Front. Bioeng. Biotechnol. 2022, 10, 846592. [Google Scholar] [CrossRef] [PubMed]
- Sidana, A.; Yadav, S.K. Recent Developments in Lignocellulosic Biomass Pretreatment with a Focus on Eco-Friendly, Non-Conventional Methods. J. Clean. Prod. 2022, 335, 130286. [Google Scholar] [CrossRef]
- Visser, E.M.; Oliveira Filho, D.; Tótola, M.R.; Martins, M.A.; Guimarães, V.M. Simultaneous Saccharification and Fermentation (SSF) of Jatropha curcas Shells: Utilization of Co-Products from the Biodiesel Production Process. Bioprocess. Biosyst. Eng. 2012, 35, 801–807. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Cara, C.; Moya, M.; Rapado, J.; Puls, J.; Castro, E.; Martín, C. Dilute Sulphuric Acid Pretreatment and Enzymatic Hydrolysis of Jatropha curcas Fruit Shells for Ethanol Production. Ind. Crops Prod. 2014, 53, 148–153. [Google Scholar] [CrossRef]
- Kumar, R.; Satlewal, A.; Sharma, S.; Kagdiyal, V.; Gupta, R.P.; Tuli, D.K.; Malhotra, R.K. Investigating Jatropha Prunings as a Feedstock for Producing Fermentable Sugars and Chemical Treatment for Process Optimization. J. Renew. Sustain. Energy 2014, 6, 033118. [Google Scholar] [CrossRef]
- Rahimi, V.; Shafiei, M.; Karimi, K. Techno-Economic Study of Castor Oil Crop Biorefinery: Production of Biodiesel without Fossil-Based Methanol and Lignoethanol Improved by Alkali Pretreatment. Agronomy 2020, 10, 1538. [Google Scholar] [CrossRef]
- Mukhopadhyay, M.; Kuila, A.; Tuli, D.K.; Banerjee, R. Enzymatic Depolymerization of Ricinus communis, a Potential Lignocellulosic for Improved Saccharification. Biomass Bioenergy 2011, 35, 3584–3591. [Google Scholar] [CrossRef]
- Deshmukh, M.; Pande, A.; Marathe, A. Different Particle Size Study of Castor Deoiled Cake for Biofuel Production with an Environmental Sustainability Perspective. Heliyon 2022, 8, e09710. [Google Scholar] [CrossRef]
- Zhong, L.; Wang, C.; Yang, G.; Chen, J.; Xu, F.; Geun Yoo, C.; Lyu, G. Rapid and Efficient Microwave-Assisted Guanidine Hydrochloride Deep Eutectic Solvent Pretreatment for Biological Conversion of Castor Stalk. Bioresour. Technol. 2022, 343, 126022. [Google Scholar] [CrossRef]
- Atelge, M.R.; Atabani, A.E.; Banu, J.R.; Krisa, D.; Kaya, M.; Eskicioglu, C.; Kumar, G.; Lee, C.; Yildiz, Y.Ş.; Unalan, S.; et al. A Critical Review of Pretreatment Technologies to Enhance Anaerobic Digestion and Energy Recovery. Fuel 2020, 270, 117494. [Google Scholar] [CrossRef]
- Millati, R.; Wikandari, R.; Ariyanto, T.; Putri, R.U.; Taherzadeh, M.J. Pretreatment Technologies for Anaerobic Digestion of Lignocelluloses and Toxic Feedstocks. Bioresour. Technol. 2020, 304, 122998. [Google Scholar] [CrossRef] [PubMed]
- Ewunie, G.A.; Yigezu, Z.D.; Morken, J. Biochemical Methane Potential of Jatropha Curcas Fruit Shell: Comparative Effect of Mechanical, Steam Explosion and Alkaline Pretreatments. Biomass Convers. Biorefinery 2022, 12, 4081–4094. [Google Scholar] [CrossRef]
- Ewunie, G.A.; Yigezu, Z.D.; Morken, J. Biochemical Methane Potential of Jatropha Press Cake: Effect of Steam Explosion Pretreatment and Co-Digestion with Crude Glycerol. J. Renew. Sustain. Energy 2020, 12, 063102. [Google Scholar] [CrossRef]
- Ewunie, G.A.; Morken, J.; Yigezu, Z.D. Alkaline and Co-Digestion Pretreatments: Process Optimization for Enhancing the Methane Yield of Jatropha Press Cake. Biomass Convers. Biorefinery 2021, 11, 971–988. [Google Scholar] [CrossRef]
- Gunaseelan, V.N. Biogas Production from Pongamia Biomass Wastes and a Model to Estimate Biodegradability from Their Composition. Waste Manag. Res. 2014, 32, 131–139. [Google Scholar] [CrossRef]
- Jabłoński, S.J.; Kułażyński, M.; Sikora, I.; Łukaszewicz, M. The Influence of Different Pretreatment Methods on Biogas Production from Jatropha curcas Oil Cake. J. Environ. Manag. 2017, 203, 714–719. [Google Scholar] [CrossRef]
- Quezada-Morales, D.L.; Campos-Guillén, J.; De Moure-Flores, F.J.; Amaro-Reyes, A.; Martínez-Martínez, J.H.; Chaparro-Sánchez, R.; Zavala-Gómez, C.E.; Flores-Macías, A.; Figueroa-Brito, R.; Rodríguez-Morales, J.A.; et al. Effect of Pretreatments on the Production of Biogas from Castor Waste by Anaerobic Digestion. Fermentation 2023, 9, 399. [Google Scholar] [CrossRef]
- IRP International Resource Panel. Global Resources Outlook; United Nations Environment Programme: Nairobi, Kenya, 2018. [Google Scholar]




| Plant Name | Oil Content 1, % (w/w) | Oil Yield, kg/ha | Web of Science Entries 2 |
|---|---|---|---|
| Jatropha (Jatropha curcas) | 20.0–60.0 [19,20,21,22] | 1590–2500 3 [23,24] | 3634 |
| Castor (Ricinus communis) | 35.0–60.0 [19,21,25] | 1188 [24] | 1025 |
| Karanja (Pongamia pinnata) | 27.0–40.0 [24,26,27] | 225–2250 [24] | 589 |
| Polanga (Calophyllum inophyllum) | 50–70 [28] | 2000 [29] | 482 |
| Rubber seed (Hevea brasiliensis) | 40.0–50.0 [24] | 80–120 [24] | 442 |
| Mahua (Madhuca longifolia) | 30.0–50.0 [8,30] | 2700 [31] | 271 |
| Neem (Azadirachta indica) | 20.0–50.0 [19,29,32] | 2000–4000 [33] | 270 |
| Tung (Vernicia fordii) | 30–40 [34] | 300–400 [35] | 200 |
| Moringa (Moringa oleifera) | 38.1–42.0 [19,36] | 2000 4 [36] | 191 |
| Kapok (Ceiba pentandra) | 25–28 [37] | 1280 [38] | 175 |
| Jojoba (Simmondsia chinensis) | 40.0–55.0 [8,29] | 1818 [39] | 114 |
| Tobacco (Nicotiana tabacum) | 30.0–49.0 [29,40,41,42] | 2200 5 [40] | 90 |
| Yellow oleander (Thevetia peruviana) | 60–65 [43] | 1750 [33] | 34 |
| Hingan (Balanites aegyptiaca) | 45.0–47.0 [44] | 1800 6 | 24 |
| Trisperma (Aleurites trisperma, also known as Reutealis trisperma) | 62.0 [19] | 400 [45] | 20 |
| Sea mango (Cerbera odollam) | 40.0–60.0 [24,46] | 1900–2400 [18] | 20 |
| Candlenut (Aleurites moluccana, also known as A. moluccanus) | 56.3–60.0 [19,47] | 3200 [47] | 16 |
| Bladderpod (Physaria fendleri, formerly known as Lesquerella fendleri) | 45.0 [48] | N.R. | 6 |
| Kurrajong (Brachychiton populneus) | 41% [49] | N.R. | 2 |
| Source | Microorganism(s) | Enzyme(s) | Application | Ref. |
|---|---|---|---|---|
| Jatropha seed cake | Pseudomonas aeruginosa | Protease, lipase | Industrial enzyme production | [126] |
| Aspergillus niger, Rhizomucor miehei | Lipase | Enzyme production | [122] | |
| Paecilomyces variotii | Cellulases | Biofuel production | [88] | |
| Scytadilium thermophilum | Xylanase | Biobleaching of paper pulp | [127] | |
| Thermoascus aurantiacus | Cellulases | Saccharification of sugarcane bagasse | [128] | |
| A. niger | Cellulase, xylanase | Biofuel production | [129] | |
| Jatropha seed husk | Bjerkandera adusta Pycnoporus sanguineus | Cellulases, xylanases | Screening of inducible enzyme activity on lignocellulosic residues | [124] |
| Castor bean waste | Penicillium simplicissimum | Lipases | Ricin detoxification and biodiesel enzyme production | [130] |
| Penicillium simplicissimum | Lipases | Biodiesel enzyme production | [131] | |
| Aspergillus spp., Emericela spp., Rhodotorula spp. | CMCase, FPase, β-glucosidase | Screening of fungal isolates for cellulase activity | [132] | |
| Pa. varoitii | Tannase, phytase | Ricin detoxification, phytate phosphate release | [65] | |
| Jojoba meal | Aspergillus spp. | Extracellular β-glucosidase | Biofuel production, fortification of T. reesei cellulases | [123] |
| Karanja seed residue | Spingomonas echinoides, Iprex lacteus | Endo- and exoglucanases, xylanase, laccase | Biofuel production | [113] |
| A. niger, Bacillus licheniformis, Acinetobacter pittii | Proteases | Enzyme production, gelatin film breakdown | [133] | |
| Moringa straw | Penicillium funiculosum, Fusarium verticillioides, Cladosporium cladosporoides | CMCase, FPase, β-glucosidase | Screening of fungal isolates for cellulase activity | [125] |
| Mahua seed cake | A. niger | Proteases | ANF detoxification | [88] |
| Enzyme | Microorganism | Substrate | SSF Length | Max. Activity (U/g Substrate) | Ref. |
|---|---|---|---|---|---|
| Lipase (EC 3.1.1.3) | P. aeruginosa | Jatropha seed cake | 120 h | 620 | [126] |
| P. simplicissimum | Castor cake | 96 h | 44.8 | [130] | |
| P. simplicissimum | Castor cake | 120 h | 155 | [131] | |
| Tannase (EC 3.1.1.20) | Pa. varoitii | Castor cake | 48 h | 2600 | [132] |
| Phytase (EC 3.1.3.8/.26) | Pa. varoitii | Castor cake | 72 h | 260 | [65] |
| Cellulase (FPase 1) (EC 3.2.x.x) | Th. aurantiacus | Jatropha seed cake | 6 days | 4.9 | [128] |
| Pa. variotii | Jatropha seed cake | 4 days | 27.3 | [88] | |
| Endoglucanse (CMCase 2) (EC 3.2.1.4) | Th. aurantiacus | Jatropha seed cake | 6 days | 124.4 | [128] |
| Aspergillus niger FGSCA733 | Jatropha seed cake | 120 h | 3974 | [128] | |
| Spingomonas echinoides | Karanja seed residue | 8 days | 16.2 | [113] | |
| Iprex lacteus | Karanja seed residue | 8 days | 49.2 | [113] | |
| Exoglucanase (EC 3.2.1.9) | S. echinoides | Karanja seed residue | 8 days | 23.4 | [113] |
| Iprex lacteus | Karanja seed residue | 8 days | 31.2 | [113] | |
| β-glucosidase (EC 3.2.1.21) | Th. aurantiacus | Jatropha seed cake | 6 days | 28.9 | [128] |
| Aspergillus sp. DHE7 | Jojoba meal | 72 h | 153 | [15] | |
| Xylanase (EC 3.2.1.8) | Scytadilium thermophilum | Jatropha seed cake | 9 days | 1455 | [127] |
| A. niger FGSCA733 | Jatropha seed cake | 48 h | 6087 | [128] | |
| S. echinoides | Karanja seed residue | 8 days | 4.8 | [113] | |
| I. lacteus | Karanja seed residue | 8 days | 16.2 | [113] | |
| Protease (EC 3.4.x.x) | P. aeruginosa PseA | Jatropha seed cake | 72 h | 1800 | [126] |
| A. niger | Mahua deoiled seed cake | 2 days | 52.5 | [88] | |
| A. niger | Karanja seed residue | 7 days | 3.7 | [133] | |
| Acinetobacter pittii | Karanja seed residue | 7 days | 1.8 | [133] | |
| B. licheniformis | Karanja seed residue | 48 h | 2.1 | [133] |
| Pretreatment Method | Material | Targeted Product (TP), Experimental Conditions (EC), and Results (R) | Ref. |
|---|---|---|---|
| Dilute-acid pretreatment | Jatropha shells | TP: ethanol EC: 121 °C, 1 h, 0.5% H2SO4; SiSF R: Yield of pretreated solids: 73%, maximum cellulose-to-ethanol conversion: 40.4%. | [158] |
| Dilute-acid pretreatment | Jatropha shells | TP: ethanol EC: SiSF applied to pretreated materials at optimized conditions (0.9% H2SO4, 178 °C, 30 min) R: ethanol yield: 72% of theoretical one | [108] |
| Dilute-acid pretreatment | Jatropha shells | TPs: glucose and ethanol EC: Temperature (110–150 °C), H2SO4 concentration (0.5–2.5%) and time (15–45 min) were optimized following a Box-Behnken design R: Optimal values: 136 °C, 1.5% H2SO4, 30 min. | [159] |
| Dilute-acid pretreatment combined with water extraction | Jatropha shells | TP: glucose EC: Water extraction followed by pretreatment with H2SO4 (0.1–1.5%) at 110–180 °C for 20–60 min; ES R: 84% cellulose conversion for pre-extracted shells and 71.5% for non-extracted shells. | [107] |
| Dilute-acid pretreatment | Jatropha pruning residues | TP: fermentable sugars and ethanol EC: H2SO4 concentration (2.5–10.0%), temperature (120–180 °C), and time (5–45 min) R: the effect of the operational conditions on xylan hydrolysis, xylose degradation, and ES of cellulose was assessed. | [160] |
| Dilute-acid pretreatment | Moringa empty pods | TP: glucose EC: 1% (w/w) H2SO4, 130–190 °C, 10–30 min; ES R: around 90% cellulose recovery in pretreatment and up to 84% conversion in ES. | [114] |
| Dilute-acid pretreatment | Moringa stem and leaves | TP: glucose and ethanol EC: 175–195 °C, 5–15 min, 0.5–4.0% (w/w) H2SO4 R: highest glucose yield (35.1 g/100 g) achieved for hydrolysis of material pretreated at 185 °C, 2% w/w acid, and 5 min. | [115] |
| Dilute-acid pretreatment | Karanja defatted kernel and hull | TP: ethanol EC: 0.5% H2SO4, 121 °C, 15 psi, 90 min, hydrolysis with 5% H2SO4 at 50 °C for 70 h. Fermentation with commercial yeast. | [64] |
| Acid pretreatment (HCl) | De-oiled jatropha waste | TP: hydrogen EC: Pretreatment with 2% (v/v) HCl at LSR 10, followed by enzymatic hydrolysis with Viscozyme and hydrogen fermentation using a hybrid immobilized cell system. | [110] |
| Alkaline pretreatment | Jatropha shells | TP: ethanol EC: NaOH (1% NaOH), 121 °C, 1 h; SiSF R: Yield of pretreated solids: 50.4%; pretreatment solubilized 63% of lignin but retained 93% of the cellulose and 99% of the xylan; maximum cellulose-to-ethanol conversion: 41%. | [158] |
| Alkaline pretreatment | De-oiled jatropha waste | TPs: glucose and lignin EC: Time (6–73 min), temperature (85–156 °C), and NaOH load (0.6–l2.4%) were optimized for maximizing lignin recovery R: Maximum lignin recovery: 93.4%; ES resulted in a glucose yield of 92.5%. | [61] |
| Alkaline pretreatment (with lime) | Jatropha press cake | TP: fermentable sugars EC: 100 °C, 1–2 h, lime dose range: 0.1–0.2 g/g, LSR: 10, 20 mL/g. R: Maximal lignin removal: 38.2%; maximal cellulose conversion: 68.9%. | [55] |
| Alkaline pretreatment | Castor plant residues | TP: ethanol and biodiesel EC: 8% (w/v) NaOH, 100 °C, 60 min, 22% solid loading; ES, ethanol fermentation R: using ethanol for producing biodiesel with castor oil was assessed. | [161] |
| Autohydrolysis | Castor pruning residues | TP: ethanol EC: 100–200 °C, 15 min R: Maximal xylan hydrolysis (77.5%) and cellulose recovery (83%); ES was 2.9-fold higher than for non-pretreated material. | [111] |
| Enzymatic pretreatment with laccases | Castor stem and leaves | TP: reducing sugars EC: Optimization of temperature (35–45 °C), pH (6.5–7.5), LSR (2–6), and enzyme load (400–600 IU/mL) for maximizing delignification R: ES of the delignified cellulose resulted in a sugar yield of 775.2 mg/g substrate. | [162] |
| Various methods | De-oiled jatropha waste | TP: fermentable sugars and hydrogen EC: Different methods (dilute-acid, alkaline, enzymatic, heat, ultrasonication) were assessed R: Viscozyme at 10% load, 10% HCl and 2.5% H2SO4 gave the highest concentrations of reducing sugars. Combined hydrolysis with acid and enzymes resulted in high hydrogen formation. | [109] |
| Heat pretreatment | De-oiled jatropha waste | TP: hydrogen EC: The effect of various combinations of heat treatments on hydrogen fermentation was assessed R: Heat-treatment proved to be necessary to inhibit methane producers. | [117] |
| Pretreatment with thionyl chloride | Castor de-oiled cake | TPs: reducing sugars and ethanol EC: Thionyl chloride at 35 °C for 25 min, enzymatic hydrolysis with T. viride cellulases. | [163] |
| Microwave-assisted deep eutectic solvent pretreatment | Castor stalk | TPs: sugars and lignin EC: Biomass suspensions in DES were irradiated at 400 W. The slurry was vacuum-filtered and the solids were washed with an acetone:water mixture R: High delignification (92%), enzymatic saccharification yields (96%), and lignin purity (up to 98%) were achieved. Good performance after recycling. | [164] |
| Ionic liquid | Jatropha hull | TP: 2,3-butanediol EC: Biomass (4 g) mixed with 100 g [BMIM]Cl at 120 °C for 1 h; the solubilized and regenerated material was hydrolyzed with H2SO4 (1.5%) at 150 °C for 30 min; the hydrolyzed was fermented with Klebsiella oxytoca R: Diol yield: 66.6%, diol productivity: 0.040 g/L h for IL-pretreated material and 0.035 g/L h for non-pretreated material. | [120] |
| Pretreatment Method | Material | Results | Ref. |
|---|---|---|---|
| Steam explosion | Jatropha press cake | Steam explosion pretreatment and co-digestion with crude glycerol were assessed to enhance methane yield. | [168] |
| Alkaline pretreatment | Jatropha press cake | Alkaline pretreatment and co-digestion with glycerol increased methane yields by 40% and 28%, respectively. | [169] |
| Alkaline pretreatment (with NaOH) at 26–32 °C | Karanja press cakes, leaves, and pod husks | Biogas yield increase: 15–22%; methane production rate increase: 20–75%. | [170] |
| Low-temperature alkaline pretreatment | Castor stem and leaves to be used for AD and ethanol production | Pretreated stems yielded 22.2 L methane or 13.6 g ethanol per kg plant biomass; pretreated leaves yielded 63 g ethanol/kg plant biomass. | [57] |
| Thermal (at 115 °C) and acidic pre-treatments | Jatropha press cake | Both methods altered the kinetics of anaerobic digestion but did not increase the biogas production efficiency. | [171] |
| Grinding, steam explosion, and alkaline pretreatments | Jatropha fruit shells | Grinding (particle size below 1 mm) enhanced the methane yield by 74%; steam explosion (160 °C, 5 min) increased the yield by 55%; alkaline pretreatment (7.32% NaOH at 36 °C for 54 h) increased the yield by 44%. | [167] |
| Enzymatic pretreatment, alkaline pretreatment, and acidic pretreatment | Castor press cake and straw (stem and leaves) | Enzymatic pretreatment (with cellulase and cellobiohydrolase), alkaline pretreatment (with NaOH), and acidic pretreatment (with HCl); AD at either room temperature or 37 °C for 55 days. | [172] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, E.; Strætkvern, K.O.; Romero-García, J.M.; Martín, C. Pretreatment and Bioconversion for Valorization of Residues of Non-Edible Oilseeds. Agronomy 2023, 13, 2196. https://doi.org/10.3390/agronomy13092196
Castro E, Strætkvern KO, Romero-García JM, Martín C. Pretreatment and Bioconversion for Valorization of Residues of Non-Edible Oilseeds. Agronomy. 2023; 13(9):2196. https://doi.org/10.3390/agronomy13092196
Chicago/Turabian StyleCastro, Eulogio, Knut Olav Strætkvern, Juan Miguel Romero-García, and Carlos Martín. 2023. "Pretreatment and Bioconversion for Valorization of Residues of Non-Edible Oilseeds" Agronomy 13, no. 9: 2196. https://doi.org/10.3390/agronomy13092196