Abstract
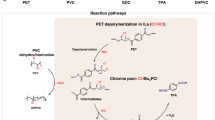
Plastics play an essential role in modern society; however, the relentless growth of their production is threatening both human health and ecosystems. As a result, there are intensive efforts in developing recycling technologies to repurpose waste plastics into the building blocks for valuable materials. Here we show a binuclear complex that can catalyse the degradation of poly(ethylene terephthalate) (PET)—the most widely used polyester globally—and a wide spectrum of other plastics including polylactic acid, polybutylene adipate terephthalate, polycaprolactone, polyurethane and Nylon 66. Inspired by hydrolases, the group of enzymes that catalyse bond cleavages with water, the present catalyst design features biomimetic Zn‒Zn sites that activate the plastic, stabilize the key intermediate and enable intramolecular hydrolysis. This synthetic catalyst delivers an activity of 36 mgPET d−1 gcatal−1 toward PET depolymerization at pH 8 and 40 °C and an activity of 577 gPET d−1 gcatal−1 at pH 13 and 90 °C for scalable PET recycling. We further demonstrate a closed-loop production of bottle-grade PET. This work presents a practical and viable solution for the sustainable management of plastics waste.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All relevant data that support the findings of this study are presented in the article and Supplementary Information. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre under deposition nos. CCDC 2082457 (Zn2L(NO3)), 2082456 (Zn2L(OH)2) and 2113556 ([Zn2L(BDC)]n). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Source data are provided with this paper.
References
Rochman, C. et al. Classify plastic waste as hazardous. Nature 494, 169–171 (2013).
Guha Roy, A. Detailing plastic pollution. Nat. Sustain 2, 654 (2019).
Geyer, R. J., Jambeck, R. & Law, K. L. Production, use, and fate of all plastics ever made. Sci. Adv. 3, e1700782 (2017).
MacLeod, M., Arp, H. P. H., Tekman, M. B. & Jahnke, A. The global threat from plastic pollution. Science 373, 61–65 (2021).
Zhang, Z. et al. Recovering waste plastics using shape-selective nano-scale reactors as catalysts. Nat. Sustain. 2, 39–42 (2019).
Jambeck, J. R. et al. Plastic waste inputs from land into the ocean. Science 347, 768–771 (2015).
George, N. & Kurian, T. Recent developments in the chemical recycling of postconsumer poly(ethylene terephthalate) waste. Ind. Eng. Chem. Res. 53, 14185–14198 (2014).
Chen, C.-C. et al. General features to enhance enzymatic activity of poly(ethylene terephthalate) hydrolysis. Nat. Catal. 4, 425–430 (2021).
Ellis, L. D. et al. Chemical and biological catalysis for plastics recycling and upcycling. Nat. Catal. 4, 539–556 (2021).
Zhu, B., Wang, D. & Wei, N. Enzyme discovery and engineering for sustainable plastic recycling. Trends Biotechnol. 40, 22–37 (2022).
Tournier, V. et al. An engineered PET depolymerase to break down and recycle plastic bottles. Nature 580, 216–219 (2020).
Tokiwa, Y. & Suzuki, T. Hydrolysis of polyesters by lipases. Nature 270, 76–78 (1977).
DelRe, C. et al. Near-complete depolymerization of polyesters with nano-dispersed enzymes. Nature 592, 558–563 (2021).
Samak, N. A. et al. Recent advances in biocatalysts engineering for polyethylene terephthalate plastic waste green recycling. Environ. Int. 145, 106144 (2020).
Yoshida, S. et al. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science 351, 1196–1199 (2016).
Štrukil, V. Highly efficient solid-state hydrolysis of waste polyethylene terephthalate by mechanochemical milling and vapor-assisted aging. ChemSusChem 14, 330–338 (2021).
Han, X. et al. Structural insight into catalytic mechanism of PET hydrolase. Nat. Commun. 8, 2106 (2017).
Joo, S. et al. Structural insight into molecular mechanism of poly(ethyleneterephthalate) degradation. Nat. Commun. 9, 382 (2018).
Austin, H. P. et al. Characterization and engineering of a plastic-degrading aromatic polyesterase. Proc. Natl Acad. Sci. USA 115, E4350–E4357 (2018).
Pinto, A. V. et al. Reaction mechanism of MHETase, a PET degrading enzyme. ACS Catal. 11, 10416–10428 (2021).
Kawai, F., Kawabata, T. & Oda, M. Current knowledge on enzymatic PET degradation and its possible application to waste stream management and other fields. Appl. Microbiol. Biotechnol. 103, 4253–4268 (2019).
Schwizer, F. et al. Artificial metalloenzymes: reaction scope and optimization strategies. Chem. Rev. 118, 142–231 (2018).
Schenk, G. et al. Binuclear metallohydrolases: complex mechanistic strategies for a simple chemical reaction. Acc. Chem. Res. 45, 1593–1603 (2012).
Wilcox, D. E. Binuclear metallohydrolases. Chem. Rev. 96, 2435–2458 (1996).
Hadler, K. S. et al. Substrate-promoted formation of a catalytically competent binuclear center and regulation of reactivity in a glycerophosphodiesterase from Enterobacter aerogenes. J. Am. Chem. Soc. 130, 14129–14138 (2008).
Pilkinqton, N. H. & Robson, R. Complexes of binucleating ligands III. Novel complexes of a macrocyclic binucleating ligand. Aust. J. Chem. 23, 2225–2236 (1970).
Dutta, B., Bag, P., Flolrke, U. & Nag, K. Dinuclear Zn(II) complexes of tetraiminodiphenol macrocycles and their interactions with carboxylate anions and amino acids. photoluminescence, equilibria, and structure. Inorg. Chem. 44, 147–157 (2005).
Kaabel, S. et al. Enzymatic depolymerization of highly crystalline polyethylene terephthalate enabled in moist-solid reaction mixtures. Proc. Natl Acad. Sci. USA 118, e2026452118 (2021).
Barth, M. et al. Effect of hydrolysis products on the enzymatic degradation of polyethylene terephthalate nanoparticles by a polyester hydrolase from Thermobifida fusca. Biochem. Eng. J. 93, 222–228 (2015).
Singh, A. et al. Techno-economic, life-cycle, and socioeconomic impact analysis of enzymatic recycling of poly(ethylene terephthalate). Joule 5, 2479–2503 (2021).
Mitić, N. et al. The catalytic mechanisms of binuclear metallohydrolases. Chem. Rev. 106, 3338–3363 (2006).
Meng, X. et al. Charge-separated metal-couple-site in NiZn alloy catalysts towards furfural hydrodeoxygenation reaction. J. Catal. 392, 69–79 (2020).
Wang, Y. et al. Zn-catalyzed ester bond cleavage: chemical degradation of polyethylene terephthalate. J. Clean. Prod. 208, 1469–1475 (2019).
Sammon, C., Yarwood, J. & Everall, N. An FT–IR study of the effect of hydrolytic degradation on the structure of thin PET films. Polym. Degrad. Stabil. 67, 149–158 (2000).
Aziz, E. F., Ottosson, N., Faubel, M., Hertel, I. V. & Winter, B. Interaction between liquid water and hydroxide revealed by core-hole de-excitation. Nature 455, 89–91 (2008).
Wolke, C. T. et al. Spectroscopic snapshots of the proton-transfer mechanism in water. Science 354, 1131–1135 (2016).
Westhues, S., Idel, J. & Klankermayer, J. Molecular catalyst systems as key enablers for tailored polyesters and polycarbonate recycling concepts. Sci. Adv. 4, eaat9669 (2018).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Ebbing, D. & Gammon, S. D. General Chemistry (Cengage, 2016).
Acknowledgements
This work was supported by the National Key R&D Program of China (grant no. 2019YFA0709200 to Z.N.), the National Natural Science Foundation of China (grant no. 22075162 to Z.N.), the Tsinghua University Initiative Scientific Research Program (grant no. 20221080067 to Z.N.) and the China Postdoctoral Science Foundation (grant no. 2021M691754 to S.Z.). The DFT calculations were performed using the Theory and Computation facility of the Centre for Functional Nanomaterials (CFN), which is a US Department of Energy Office of Science User Facility, at Brookhaven National Laboratory under Contract No. DE-SC0012704 (to P.L.). We acknowledge the BL14W1 station of the Shanghai Synchrotron Radiation Facility (SSRF) and the 4B9A station of the Beijing Synchrotron Radiation Facility (BSRF) for the collection of XAFS data.
Author information
Authors and Affiliations
Contributions
Z.N. conceptualized and guided this work. Z.N. and S.Z. designed the experiments. S.Z., Q.H. and Y.-X.Z. performed the experiments and contributed equally to this work. H.G. and P.L. performed the DFT calculations. X.Z. carried out the production and characterization of rPET using recycled PTA. Y.W. and M.S. carried out the EXAFS measurements and analysis. S.G. and J.Z. performed the STEM measurements. Z.N., S.Z., Q.H., Y.-X.Z., H.G. and P.L. wrote the paper. All authors participated in the data analysis and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
Z.N., S.Z., Q.H. and Y.-X.Z. have filed a PCT patent (China Patent application no. PCT/CN2021/124875). X.Z. is an employee of Sinopec Yizheng Chemical Fibre Co., Ltd. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Rey-Ting Guo and Thomas Maskow for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Structures of binuclear zinc complexes.
a‒c, Experimental (gray) and simulated (light gray) SCXRD patterns of Zn2L(NO3)2 (a), Zn2L(OH)2 (b), and [Zn2L(BDC)]n (c). The insets show the optical images of the single crystals and the corresponding simplified crystal structures (green: Zn; blue: N; red: O; gray: C). d, Hydrogen bonding network in Zn2L(OH)2 as resolved by SCXRD. Free solvent molecules and hydrogen atoms are omitted for clarity, except for the hydrogen atoms in the coordinated groups and hydrogen bonds.
Extended Data Fig. 2 The PET hydrolysis mechanism proposed based on experimental and DFT results.
The mechanism highlights the importance of co-operative substrate-binding on the adjacent zinc sites and the subsequent formation of six-membered (Zn-O-C-O-Zn-μ2-O) intermediate. Ball-and-stick models are given near the corresponding skeleton formulas (green: Zn; blue: N; red: O; gray: C; white: H). Ethylene glycol dibenzoate (EGD) is employed as the ester substrate for simplicity. EGM and BzO represent ethylene glycol monobenzoate and benzoate, respectively.
Extended Data Fig. 3 Performance evaluation of binuclear zinc catalysts.
a, The hydrolysis kinetics of crystalline PET granules (38%) over Zn2L(OH)2 with equivalent moles of free NO3‒ and Zn2L(NO3)2 with equivalent moles of free OH‒. Reaction conditions: 1.0 mM Zn2L(NO3)2 or Zn2L(OH)2, 2.0 mM NaOH or NaNO3, 10 mg crystalline PET granules, 60 °C. Note that the induction period was curtailed when the axial ligands were hydroxyl groups, in spite of the same feeding amount in the two cases. b, The performance of Zn2/C under different pH values and temperatures, where the areas of the circles represent the magnitude of the specific activity. The specific activity of the Zn2/C at pH 8 and 40 °C is 36 mgPET d−1 gcatal−1. The specific activities were calculated at 80% conversion of PET in 10 mL of NaOH aqueous solution containing 10 mg of PET and 4 mg of Zn2L(NO3)2 supported on carbon. c, Thermogravimetric analysis (TGA) of Zn2L(NO3)2, showing that the binuclear catalyst was stable below 340 °C. d, The hydrolysis kinetics of crystalline PET granules (38%) over Zn2/C, Zn(OAc)2, and blank control at pH 13 and 90 °C. Reaction conditions: 50 mL of NaOH aqueous solution (pH 13) containing 50 g of crystalline PET (38%) and 5 mg or 50 mg of Zn2L(NO3)2 supported on carbon or 35 mg of Zn(OAc)2 (Methods). All the error bars in a and d represent the standard deviations for three independent measurements and the hollow squares indicate mean values. The shading in the d represents the error range.
Extended Data Fig. 4 X-ray adsorption spectroscopy of Zn2L(OH)2 during reaction.
a‒c, XANES spectra (a), Fourier transforms of EXAFS spectra (b), and EXAFS in k-space spectra (c) of the binuclear Zn catalyst before and during the reaction. EXAFS spectra in R-space indicate an increased coordination number of zinc during the reaction.
Extended Data Fig. 5 The variation of coordination geometries in response to the change of coordination number of the metal sites.
a, Ball-and-stick model of Zn2L(NO3)2 as resolved by SCXRD, where hydrogen atoms are omitted for clarity. b, The relative position of disordered Zn atoms and N4O2 plane. Lavender: pentacoordinated; green: hexacoordinated. Atom–plane distances: Zn1, 0.123 Å; Zn1A, 0.391 Å; Zn2, 0.090 Å; Zn2A, 0.508 Å. c‒d, Pentacoordinated (c) and hexacoordinated (d) configuration of Zn atoms in Zn2L(NO3)2. In the case of pentacoordinate, the axial positions of Zn atoms are occupied by nitrate ions and form distorted square pyramidal geometry. The two Zn atoms distinctly lie out of N4O2 plane, with atom–plane distances of 0.391 Å and 0.508 Å, and elongated Zn···Zn distance of 3.249(5) Å, respectively. In the case of hexacoordinate, the two Zn atoms take distorted octahedral configuration, with additional methanol molecules coordinated to the Zn atoms. The Zn–plane distances are negligible 0.123 Å and 0.090 Å, while Zn···Zn distance is shortened to 3.153(3) Å. This statistical distribution in the crystal structure of Zn2L(NO3)2 indicates that the pentacoordinated Zn atoms could form a bond to another molecule and turn into hexacoordinate structure.
Extended Data Fig. 6 Post-reaction characterization of Zn2/C.
a‒d, HADDF-STEM image (scale bar: 5 nm) (a), XANES spectra (b), Fourier transforms of EXAFS spectra (c), and EXAFS spectra in k space (d) of the Zn K-edge of spent Zn2/C. The XAFS spectra of the fresh Zn2/C were also presented in b‒d for references. These results indicate the structure of the spent Zn2/C remained the same to its original form.
Extended Data Fig. 7 Crystallinity of different PET materials.
a‒b, XRD patterns (a) and differential scanning calorimetry (DSC) analysis (b) of amorphous film (Goodfellow Ltd, ES301445), crystalline granule (Macklin, P875573), dyed water bottle (Coca-cola, USA), and cloth fiber (online purchase). Insets in a show the images of different PET materials. The corresponding crystallinity was listed in parentheses in b.
Extended Data Fig. 8 PET recycling using Zn2/C.
a‒b, Flow diagram (a) and representative photos (b) of the PET recycling process, where TPA-Na2 and EG represent disodium terephthalate and ethylene glycol, respectively. c‒d, The purity of the recycled pure terephthalic acid (PTA) was checked by 1H NMR (c) and high-performance liquid chromatography (d). Both confirmed that the purity of terephthalic acid (TPA) was above 99% according to the national standard GB/T 30921.1. e, Comparison of rPET made from the recycled PTA with the PET made from virgin PTA.
Extended Data Fig. 9 Techno-economic analysis based on a capability of 100 thousand tons of PET waste per year.
a, Histogram analysis of the cost and revenue for Zn2/C-catalysed PET recycling, showing an annual profit of 26.0 million USD on 0.1 megatons of PET waste. b, Simplified flow diagram of the PET recycling process. The raw material inputs are shown in blue, and the products are shown in red. c, Simplified economic summary. More detailed analysis is provided in the Supplementary Information.
Extended Data Fig. 10 The depolymerization of different PET sources and various polyesters and polyamide with and without Zn2/C at pH 13 and 60 °C.
Reaction conditions: 10 mL of NaOH aqueous solution (pH 13), 0.32 g of polyesters or polyamide and 2 mg of Zn2L(NO3)2 supported on carbon. Reaction time: Carpet (60 h), fiber (50 h), dyed bottle (50 h), waste bottle (50 h), amorphous (38 h), crystalline granule (50 h), Nylon 66 (6 day), PBT (12 day), PC (14 day), PBA (38 h), P3/4HB (18 h), PHB (27 h), PGA (10 h), PBS (12 h), PEF (7 h), PU (2 h), PCL (16 h), PLA (18 h), and PBAT (16 h). All the error bars in this figure represent the standard deviations for three independent measurements and the bars indicate mean values.
Supplementary information
Supplementary Information
Characterizations, economic analysis, DFT calculations, Supplementary Tables 1‒5 and References.
Source data
Source Data Fig. 2
Source Data Fig. 2.
Source Data Fig. 3
Source Data Fig. 3.
Source Data Fig. 4
Source Data Fig. 4.
Source Data Fig. 5
Source Data Fig. 5.
Source Data Extended Data Fig. 1
Source Data Extended Data Fig. 1.
Source Data Extended Data Fig. 3
Source Data Extended Data Fig. 3.
Source Data Extended Data Fig. 4
Source Data Extended Data Fig. 4.
Source Data Extended Data Fig. 6
Source Data Extended Data Fig. 6.
Source Data Extended Data Fig. 7
Source Data Extended Data Fig. 7.
Source Data Extended Data Fig. 8
Source Data Extended Data Fig. 8.
Source Data Extended Data Fig. 10
Source Data Extended Data Fig. 10.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, S., Hu, Q., Zhang, YX. et al. Depolymerization of polyesters by a binuclear catalyst for plastic recycling. Nat Sustain 6, 965–973 (2023). https://doi.org/10.1038/s41893-023-01118-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-023-01118-4
This article is cited by
-
Grave-to-cradle photothermal upcycling of waste polyesters over spent LiCoO2
Nature Communications (2024)
-
A general strategy for recycling polyester wastes into carboxylic acids and hydrocarbons
Nature Communications (2024)
-
Photogenerated outer electric field induced electrophoresis of organic nanocrystals for effective solid-solid photocatalysis
Nature Communications (2024)
-
Fabrication of Silane and Desulfurization Ash Composite Modified Polyurethane and Its Interfacial Binding Mechanism
Journal of Wuhan University of Technology-Mater. Sci. Ed. (2024)
-
Catalytic recycling of polyesters via a binuclear catalyst
Science China Chemistry (2024)